Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway
- PMID: 33769187
- PMCID: PMC8007154
- DOI: 10.1080/19490976.2021.1902718
Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway
Abstract
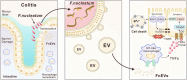
Microbial factors that mediate microbes-host interaction in ulcerative colitis (UC), a chronic disease seriously affecting human health, are not fully known. The emerging oncobacterium Fusobacterium nucleatum (Fn) secretes extracellular vesicles carrying several types of harmful molecules in the intestine which can alter microbes-host interaction, especially the epithelial homeostasis in UC. However, the mechanism is not yet clear. Previously, we isolated EVs by the ultracentrifugation of Fn culture media and characterized them as the potent inducer of pro-inflammatory cytokines. Here, we examined the mechanism in detail. We found that in macrophage/Caco-2 co-cultures, FnEVs significantly promoted epithelial barrier loss and oxidative stress damage, which are related to epithelial necroptosis caused by the activation of receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3). Furthermore, FnEVs promoted the migration of RIPK1 and RIPK3 into necrosome in Caco2 cells. Notably, these effects were reversed by TNF-α neutralizing antibody or Necrostatin-1 (Nec-1), a RIPK1 inhibitor. This suggested that FADD-RIPK1-caspase-3 signaling is involved in the process. Moreover, the observed effects were verified in the murine colitis model treated with FnEVs or by adoptive transfer of FnEVs-trained macrophages. In conclusion, we propose that RIPK1-mediated epithelial cell death promotes FnEVs-induced gut barrier disruption in UC and the findings can be used as the basis to further investigate this disease.
Keywords: Fusobacterium nucleatum; extracellular vesicles; macrophages; necroptosis; oncobacterium; ulcerative colitis.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
