Pathogenesis and shedding of Usutu virus in juvenile chickens
- PMID: 33769213
- PMCID: PMC8043533
- DOI: 10.1080/22221751.2021.1908850
Pathogenesis and shedding of Usutu virus in juvenile chickens
Abstract
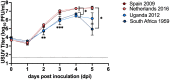
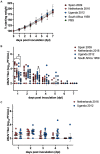
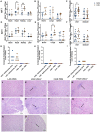
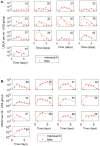
Usutu virus (USUV; family: Flaviviridae, genus: Flavivirus), is an emerging zoonotic arbovirus that causes severe neuroinvasive disease in humans and has been implicated in the loss of breeding bird populations in Europe. USUV is maintained in an enzootic cycle between ornithophilic mosquitos and wild birds. As a member of the Japanese encephalitis serocomplex, USUV is closely related to West Nile virus (WNV) and St. Louis encephalitis virus (SLEV), both neuroinvasive arboviruses endemic in wild bird populations in the United States. An avian model for USUV is essential to understanding zoonotic transmission. Here we describe the first avian models of USUV infection with the development of viremia. Juvenile commercial ISA Brown chickens were susceptible to infection by multiple USUV strains with evidence of cardiac lesions. Juvenile chickens from two chicken lines selected for high (HAS) or low (LAS) antibody production against sheep red blood cells showed markedly different responses to USUV infection. Morbidity and mortality were observed in the LAS chickens, but not HAS chickens. LAS chickens had significantly higher viral titers in blood and other tissues, as well as oral secretions, and significantly lower development of neutralizing antibody responses compared to HAS chickens. Mathematical modelling of virus-host interactions showed that the viral clearance rate is a stronger mitigating factor for USUV viremia than neutralizing antibody response in this avian model. These chicken models provide a tool for further understanding USUV pathogenesis in birds and evaluating transmission dynamics between avian hosts and mosquito vectors.
Keywords: Usutu virus; avian model; juvenile chicken; pathogenesis; virus-host interactions.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Cross-Protection between West Nile Virus and Emerging Flaviviruses in Wild Birds.Am J Trop Med Hyg. 2024 Dec 31;112(3):657-662. doi: 10.4269/ajtmh.24-0363. Print 2025 Mar 5. Am J Trop Med Hyg. 2024. PMID: 39742522 Free PMC article.
-
Effect of blood source on vector competence of Culex pipiens biotypes for Usutu virus.Parasit Vectors. 2021 Apr 8;14(1):194. doi: 10.1186/s13071-021-04686-6. Parasit Vectors. 2021. PMID: 33832527 Free PMC article.
-
Cross-protection against St. Louis encephalitis virus and Usutu virus by West Nile virus convalescent plasma.Virology. 2025 Jul;608:110555. doi: 10.1016/j.virol.2025.110555. Epub 2025 Apr 18. Virology. 2025. PMID: 40273513
-
Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges.Viruses. 2024 Apr 12;16(4):599. doi: 10.3390/v16040599. Viruses. 2024. PMID: 38675940 Free PMC article. Review.
-
In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus.Viruses. 2020 Sep 30;12(10):1116. doi: 10.3390/v12101116. Viruses. 2020. PMID: 33008141 Free PMC article. Review.
Cited by
-
Interactions between avian viruses and skin in farm birds.Vet Res. 2024 Apr 26;55(1):54. doi: 10.1186/s13567-024-01310-0. Vet Res. 2024. PMID: 38671518 Free PMC article. Review.
-
North American House Sparrows Are Competent for Usutu Virus Transmission.mSphere. 2022 Dec 21;7(6):e0029522. doi: 10.1128/msphere.00295-22. Epub 2022 Nov 1. mSphere. 2022. PMID: 36317895 Free PMC article.
-
Dose and strain dependent lethality of Usutu virus in an Ifnar-/- mouse model.Npj Viruses. 2025 Jan 28;3(1):6. doi: 10.1038/s44298-025-00089-x. Npj Viruses. 2025. PMID: 40295862 Free PMC article.
-
Evidence for overwintering and autochthonous transmission of Usutu virus to wild birds following its redetection in the United Kingdom.Transbound Emerg Dis. 2022 Nov;69(6):3684-3692. doi: 10.1111/tbed.14738. Epub 2022 Oct 25. Transbound Emerg Dis. 2022. PMID: 36217722 Free PMC article.
-
Experimental infections in red-legged partridges reveal differences in host competence between West Nile and Usutu virus strains from Southern Spain.Front Cell Infect Microbiol. 2023 Jun 15;13:1163467. doi: 10.3389/fcimb.2023.1163467. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37396301 Free PMC article.
References
-
- Williams MC, Simpson DIH, Haddow AJ, et al. . The isolation of West Nile virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (Theobald) in the entebbe area of Uganda. Ann Trop Med Parasitol. 1964;58(3):367–374. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
