Treatment and prevention of lipoprotein(a)-mediated cardiovascular disease: the emerging potential of RNA interference therapeutics
- PMID: 33769464
- PMCID: PMC8953457
- DOI: 10.1093/cvr/cvab100
Treatment and prevention of lipoprotein(a)-mediated cardiovascular disease: the emerging potential of RNA interference therapeutics
Abstract
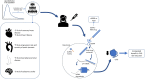
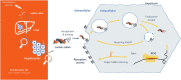
Lipid- and lipoprotein-modifying therapies have expanded substantially in the last 25 years, resulting in reduction in the incidence of major adverse cardiovascular events. However, no specific lipoprotein(a) [Lp(a)]-targeting therapy has yet been shown to reduce cardiovascular disease risk. Many epidemiological and genetic studies have demonstrated that Lp(a) is an important genetically determined causal risk factor for coronary heart disease, aortic valve disease, stroke, heart failure, and peripheral vascular disease. Accordingly, the need for specific Lp(a)-lowering therapy has become a major public health priority. Approximately 20% of the global population (1.4 billion people) have elevated levels of Lp(a) associated with higher cardiovascular risk, though the threshold for determining 'high risk' is debated. Traditional lifestyle approaches to cardiovascular risk reduction are ineffective at lowering Lp(a). To address a lifelong risk factor unmodifiable by non-pharmacological means, Lp(a)-lowering therapy needs to be safe, highly effective, and tolerable for a patient population who will likely require several decades of treatment. N-acetylgalactosamine-conjugated gene silencing therapeutics, such as small interfering RNA (siRNA) and antisense oligonucleotide targeting LPA, are ideally suited for this application, offering a highly tissue- and target transcript-specific approach with the potential for safe and durable Lp(a) lowering with as few as three or four doses per year. In this review, we evaluate the causal role of Lp(a) across the cardiovascular disease spectrum, examine the role of established lipid-modifying therapies in lowering Lp(a), and focus on the anticipated role for siRNA therapeutics in treating and preventing Lp(a)-related disease.
Keywords: Cardiovascular disease; Lipoprotein(a); RNA interference; Therapeutics; Valvular disease.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Berg K. A new serum type system in man-the Lp system. Acta Pathol Microbiol Scand 1963;59:369–382. - PubMed
-
- Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation 2008;117:176–184. - PubMed
-
- O'Donoghue M, Giugliano R, Keech A, Kanevsky E, Im K, Pineda AL, Somaratne R, Sever P, Pederson T, Sabatine M. Lipoprotein(a), PCSK9 Inhibition and cardiovascular risk: insights from the Fourier trial. Atherosclerosis 2018;275:e9–e10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

