Proteomics characterization of mitochondrial-derived vesicles under oxidative stress
- PMID: 33769614
- PMCID: PMC8252493
- DOI: 10.1096/fj.202002151R
Proteomics characterization of mitochondrial-derived vesicles under oxidative stress
Abstract
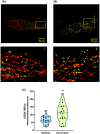
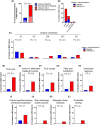
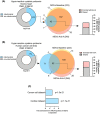
Mitochondria share attributes of vesicular transport with their bacterial ancestors given their ability to form mitochondrial-derived vesicles (MDVs). MDVs are involved in mitochondrial quality control and their formation is enhanced with stress and may, therefore, play a potential role in mitochondrial-cellular communication. However, MDV proteomic cargo has remained mostly undefined. In this study, we strategically used an in vitro MDV budding/reconstitution assay on cardiac mitochondria, followed by graded oxidative stress, to identify and characterize the MDV proteome. Our results confirmed previously identified cardiac MDV markers, while also revealing a complete map of the MDV proteome, paving the way to a better understanding of the role of MDVs. The oxidative stress vulnerability of proteins directed the cargo loading of MDVs, which was enhanced by antimycin A (Ant-A). Among OXPHOS complexes, complexes III and V were found to be Ant-A-sensitive. Proteins from metabolic pathways such as the TCA cycle and fatty acid metabolism, along with Fe-S cluster, antioxidant response proteins, and autophagy were also found to be Ant-A sensitive. Intriguingly, proteins containing hyper-reactive cysteine residues, metabolic redox switches, including professional redox enzymes and those that mediate iron metabolism, were found to be components of MDV cargo with Ant-A sensitivity. Last, we revealed a possible contribution of MDVs to the formation of extracellular vesicles, which may indicate mitochondrial stress. In conclusion, our study provides an MDV proteomics signature that delineates MDV cargo selectivity and hints at the potential for MDVs and their novel protein cargo to serve as vital biomarkers during mitochondrial stress and related pathologies.
Keywords: hyper-reactive cysteine residues; mitochondrial iron transport; mitochondrial quality control; mitochondrial stress; mitochondrial-derived vesicle proteome.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
-
- Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437(7057):422‐425. - PubMed
-
- Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other Gram‐negative bacteria. Microbiology. 1999;145(8):2051‐2060. - PubMed
-
- Soubannier V, McLelland G‐L, Zunino R, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22(2):135‐141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

