Essential properties and pitfalls of colorimetric Reverse Transcription Loop-mediated Isothermal Amplification as a point-of-care test for SARS-CoV-2 diagnosis
- PMID: 33771097
- PMCID: PMC7996115
- DOI: 10.1186/s10020-021-00289-0
Essential properties and pitfalls of colorimetric Reverse Transcription Loop-mediated Isothermal Amplification as a point-of-care test for SARS-CoV-2 diagnosis
Abstract
Background: SARS-CoV-2 Reverse Transcription Loop-mediated Isothermal Amplification (RT-LAMP) colorimetric detection is a sensitive and specific point-of-care molecular biology technique used to detect the virus in only 30 min. In this manuscript we have described a few nuances of the technique still not properly described in the literature: the presence of three colors clusters; the correlation of the viral load with the color change; and the importance of using an internal control to avoid false-negative results.
Methods: To achieve these findings, we performed colorimetric RT-LAMP assays of 466 SARS-CoV-2 RT-qPCR validated clinical samples, with color quantification measured at 434 nm and 560 nm.
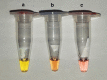
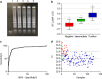
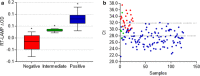
Results: First we determinate a sensitivity of 93.8% and specificity of 90.4%. In addition to the pink (negative) and yellow (positive) produced colors, we report for the first time the presence of an orange color cluster that may lead to wrong diagnosis. We also demonstrated using RT-qPCR and RT-LAMP that low viral loads are related to Ct values > 30, resulting in orange colors. We also demonstrated that the diagnosis of COVID-19 by colorimetric RT-LAMP is efficient until the fifth symptoms day when the viral load is still relatively high.
Conclusion: This study reports properties and indications for colorimetric RT-LAMP as point-of-care for SARS-CoV-2 diagnostic, reducing false results, interpretations and optimizing molecular diagnostics tests application.
Keywords: Point of Care; RT-LAMP; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ahn SJ, Baek YH, Lloren KKS, Choi WS, Jeong JH, Antigua KJC, Kwon HI, Park SJ, Kim EH, Kim YI, Si YJ, Hong SB, Shin KS, Chun S, Choi YK, Song MS. Rapid and simple colorimetric detection of multiple influenza viruses infecting humans using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. BMC Infect Dis. 2019;19(1):1–12. doi: 10.1186/s12879-019-4277-8. - DOI - PMC - PubMed
-
- Ali Z, Aman R, Mahas A, Rao GS, Tehseen M, Marsic T, Salunke R, Subudhi AK, Hala SM, Hamdan SM, Pain A, Alofi FS, Alsomali A, Hashem AM, Khogeer A, Almontashiri NAM, Abedalthagafi M, Hassan N, Mahfouz MM. ISCAN: an RT-LAMP-Coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020;288:198129. doi: 10.1016/j.virusres.2020.198129. - DOI - PMC - PubMed
-
- Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Notomi T, Kevadiya B, Thakor A. Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology. 2020;9(8):182. doi: 10.3390/biology9080182. - DOI - PMC - PubMed
-
- Chembio Diagnostic System, DPP® COVID-19 IgM/IgG System, 2020, 14 Apr 2020. https://www.fda.gov/media/136963/download, 26th Feb 2021.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

