Cost-impact analysis of baroreflex activation therapy in chronic heart failure patients in the United States
- PMID: 33771104
- PMCID: PMC7995802
- DOI: 10.1186/s12872-021-01958-y
Cost-impact analysis of baroreflex activation therapy in chronic heart failure patients in the United States
Abstract
Background: The study evaluated the cost of baroreflex activation therapy plus guideline directed therapy (BAT + GDT) compared to GDT alone for HF patients with reduced ejection fraction and New York Heart Association Class III or II (with a recent history of III). Baroreflex activation therapy (BAT) is delivered by an implantable device that stimulates the baroreceptors through an electrode attached to the outside of the carotid artery, which rebalances the autonomic nervous system to regain cardiovascular (CV) homeostasis. The BeAT-HF trial evaluated the safety and effectiveness of BAT.
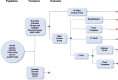
Methods: A cost impact model was developed from a U.S. health care payer or integrated delivery network perspective over a 3-year period for BAT + GDT versus GDT alone. Expected costs were calculated by utilizing 6-month data from the BeAT-HF trial and existing literature. HF hospitalization rates were extrapolated based on improvement in NT-proBNP.
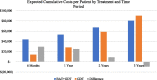
Results: At baseline the expected cost of BAT + GDT were $29,526 per patient more than GDT alone due to BAT device and implantation costs. After 3 years, the predicted cost per patient was $9521 less expensive for BAT + GDT versus GDT alone due to lower rates of significant HF hospitalizations, CV non-HF hospitalizations, and resource intensive late-stage procedures (LVADs and heart transplants) among the BAT + GDT group.
Conclusions: BAT + GDT treatment becomes less costly than GDT alone beginning between years 1 and 2 and becomes less costly cumulatively between years 2 and 3, potentially providing significant savings over time. As additional BeAT-HF trial data become available, the model can be updated to show longer term effects.
Keywords: Baroreflex activation therapy; Costs; Economic; Heart failure.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
An early analysis of cost-utility of baroreflex activation therapy in advanced chronic heart failure in Germany.BMC Cardiovasc Disord. 2018 Aug 9;18(1):163. doi: 10.1186/s12872-018-0898-x. BMC Cardiovasc Disord. 2018. PMID: 30092774 Free PMC article.
-
Baroreflex Activation Therapy in Patients With Heart Failure With Reduced Ejection Fraction.J Am Coll Cardiol. 2020 Jul 7;76(1):1-13. doi: 10.1016/j.jacc.2020.05.015. J Am Coll Cardiol. 2020. PMID: 32616150 Clinical Trial.
-
Baroreflex activation therapy in patients with heart failure and a reduced ejection fraction: Long-term outcomes.Eur J Heart Fail. 2024 Apr;26(4):1051-1061. doi: 10.1002/ejhf.3232. Epub 2024 Apr 12. Eur J Heart Fail. 2024. PMID: 38606555 Clinical Trial.
-
[Baroreflex activation therapy. A novel interventional approach to treat heart failure with reduced ejection fraction].Herz. 2015 Nov;40(7):959-65. doi: 10.1007/s00059-015-4361-9. Herz. 2015. PMID: 26525523 Review. German.
-
Baroreflex Activation Therapy in Heart Failure With Reduced Ejection Fraction: Available Data and Future Perspective.Curr Heart Fail Rep. 2016 Apr;13(2):71-6. doi: 10.1007/s11897-016-0286-8. Curr Heart Fail Rep. 2016. PMID: 26879389 Review.
Cited by
-
A Comparative Review of Vagal Nerve Stimulation Versus Baroreceptor Activation Therapy in Cardiac Diseases.Cureus. 2023 Jun 24;15(6):e40889. doi: 10.7759/cureus.40889. eCollection 2023 Jun. Cureus. 2023. PMID: 37492836 Free PMC article. Review.
-
Neuromodulation devices for heart failure.Eur Heart J Suppl. 2022 Aug 17;24(Suppl E):E12-E27. doi: 10.1093/eurheartjsupp/suac036. eCollection 2022 Sep. Eur Heart J Suppl. 2022. PMID: 35991619 Free PMC article.
-
Efficacy and safety of baroreflex activation therapy for heart failure with reduced ejection fraction: systematic review.ESC Heart Fail. 2023 Oct;10(5):2760-2772. doi: 10.1002/ehf2.14473. Epub 2023 Jul 31. ESC Heart Fail. 2023. PMID: 37522644 Free PMC article.
-
Neurocardiology: translational advancements and potential.J Physiol. 2025 Mar;603(7):1729-1779. doi: 10.1113/JP284740. Epub 2024 Sep 27. J Physiol. 2025. PMID: 39340173 Free PMC article. Review.
References
-
- CDC. Heart Failure Fact Sheet 2019. Available from: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm.
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Cardiac Fail. 2016;22(9):659–669. doi: 10.1016/j.cardfail.2016.07.001. - DOI - PubMed
-
- Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 Appropriate Use Criteria for Implantable Cardioverter-Defibrillators and Cardiac Resynchronization Therapy: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61(12):1318–1368. doi: 10.1016/j.jacc.2012.12.017. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous