Drug-drug interactions in subjects enrolled in SWOG trials of oral chemotherapy
- PMID: 33771105
- PMCID: PMC7995697
- DOI: 10.1186/s12885-021-08050-w
Drug-drug interactions in subjects enrolled in SWOG trials of oral chemotherapy
Abstract
Background: Patients with cancer are at increased risk of drug-drug interactions (DDI), which can increase treatment toxicity or decrease efficacy. It is especially important to thoroughly screen DDI in oncology clinical trial subjects to ensure trial subject safety and data accuracy. This study determined the prevalence of potential DDI involving oral anti-cancer trial agents in subjects enrolled in two SWOG clinical trials.
Methods: Completed SWOG clinical trials of commercially available agents with possible DDI that had complete concomitant medication information available at enrollment were included. Screening for DDI was conducted through three methods: protocol-guided screening, Lexicomp® screening, and pharmacist determination of clinical relevance. Descriptive statistics were calculated.
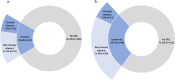
Results: SWOG trials S0711 (dasatinib, n = 83) and S0528 (everolimus/lapatinib, n = 84) were included. Subjects received an average of 6.6 medications (standard deviation = 4.9, range 0-29) at enrollment. Based on the clinical trial protocols, at enrollment 18.6% (31/167) of subjects had a DDI and 12.0% (20/167) had a DDI that violated a protocol exclusion criterion. According to Lexicomp®, 28.7% of subjects (48/167) had a DDI classified as moderate or worse, whereas pharmacist review indicated that 7.2% of subjects (12/167) had a clinically relevant interaction. The majority of clinically relevant DDI identified were due to the coadministration of acid suppression therapies with dasatinib (83.3%, 10/12).
Conclusions: The high DDI prevalence in subjects enrolled on SWOG clinical trials, including a high prevalence that violate trial exclusion criteria, support the need for improved processes for DDI screening to ensure trial subject safety and trial data accuracy.
Keywords: Oncology clinical trial drug interaction.
Conflict of interest statement
LAM and DLH are working with PEPID LLC to create a drug interaction screening tool for use during clinical trial enrollment. PEPID LLC was not involved in the design, conduct, analysis, or sponsorship of this trial, and had no contribution to the writing of this manuscript. This work was accepted for poster presentation at the 2019 American College of Clinical Pharmacology Annual Meeting. The SWOG Statistics and Data Management Center (CA180819) and SWOG Network Group Operations Center of the NCTN (CA180888) provided the retrospective data to the co-authors of this study.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

