Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation
- PMID: 33771213
- PMCID: PMC8004413
- DOI: 10.1186/s13073-021-00863-5
Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation
Abstract
Background: Neuroinflammation and immune responses occurring minutes to hours after stroke are associated with brain injury after acute ischemic stroke (AIS). PPARγ coactivator-1α (PGC-1α), as a master coregulator of gene expression in mitochondrial biogenesis, was found to be transiently upregulated in microglia after AIS. However, the role of microglial PGC-1α in poststroke immune modulation remains unknown.
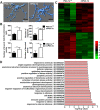
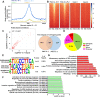
Methods: PGC-1α expression in microglia from human and mouse brain samples following ischemic stroke was first determined. Subsequently, we employed transgenic mice with microglia-specific overexpression of PGC-1α for middle cerebral artery occlusion (MCAO). The morphology and gene expression profile of microglia with PGC-1α overexpression were evaluated. Downstream inflammatory cytokine production and NLRP3 activation were also determined. ChIP-Seq analysis was performed to detect PGC-1α-binding sites in microglia. Autophagic and mitophagic activity was further monitored by immunofluorescence staining. Unc-51-like autophagy activating kinase 1 (ULK1) expression was evaluated under the PGC-1α interaction with ERRα. Finally, pharmacological inhibition and genomic knockdown of ULK1 were performed to estimate the role of ULK1 in mediating mitophagic activity after ischemic stroke.
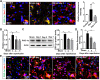
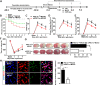
Results: PGC-1α expression was shortly increased after ischemic stroke, not only in human brain samples but also in mouse brain samples. Microglia-specific PGC-1α overexpressing mice exhibited significantly decreased neurologic deficits after ischemic injury, with reduced NLRP3 activation and proinflammatory cytokine production. ChIP-Seq analysis and KEGG pathway analysis revealed that mitophagy was significantly enhanced. PGC-1α significantly promoted autophagic flux and induced autolysosome formation. More specifically, the autophagic clearance of mitochondria was enhanced by PGC-1α regulation, indicating the important role of mitophagy. Pharmacological inhibition or knockdown of ULK1 expression impaired autophagic/mitophagic activity, thus abolishing the neuroprotective effects of PGC-1α.
Conclusions: Mechanistically, in AIS, PGC-1α promotes autophagy and mitophagy through ULK1 and reduces NLRP3 activation. Our findings indicate that microglial PGC-1α may be a promising therapeutic target for AIS.
Keywords: Ischemic stroke; Microglia; Neuroinflammation; PGC-1α.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

