Prion-like C-Terminal Domain of TDP-43 and α-Synuclein Interact Synergistically to Generate Neurotoxic Hybrid Fibrils
- PMID: 33771571
- PMCID: PMC8085152
- DOI: 10.1016/j.jmb.2021.166953
Prion-like C-Terminal Domain of TDP-43 and α-Synuclein Interact Synergistically to Generate Neurotoxic Hybrid Fibrils
Abstract
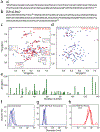
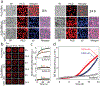
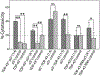
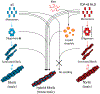
Aberrant aggregation and amyloid formation of tar DNA binding protein (TDP-43) and α-synuclein (αS) underlie frontotemporal dementia (FTD) and Parkinson's disease (PD), respectively. Amyloid inclusions of TDP-43 and αS are also commonly co-observed in amyotrophic lateral sclerosis (ALS), dementia with Lewy bodies (DLB) and Alzheimer disease (AD). Emerging evidence from cellular and animal models show colocalization of the TDP-43 and αS aggregates, raising the possibility of direct interactions and co-aggregation between the two proteins. In this report, we set out to answer this question by investigating the interactions between αS and prion-like pathogenic C-terminal domain of TDP-43 (TDP-43 PrLD). PrLD is an aggregation-prone fragment generated both by alternative splicing as well as aberrant proteolytic cleavage of full length TDP-43. Our results indicate that two proteins interact in a synergistic manner to augment each other's aggregation towards hybrid fibrils. While monomers, oligomers and sonicated fibrils of αS seed TDP-43 PrLD monomers, TDP-43 PrLD fibrils failed to seed αS monomers indicating selectivity in interactions. Furthermore, αS modulates liquid droplets formed by TDP-43 PrLD and RNA to promote insoluble amyloid aggregates. Importantly, the cross-seeded hybrid aggregates show greater cytotoxicity as compared to the individual homotypic aggregates suggesting that the interactions between the two proteins have a discernable impact on cellular functions. Together, these results bring forth insights into TDP-43 PrLD - αS interactions that could help explain clinical and pathological presentations in patients with co-morbidities involving the two proteins.
Keywords: TDP-43; amyloid; fibrillization; liquid–liquid phase separation; α-synuclein.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Soto C & Estrada LD (2008). Protein misfolding and neurodegeneration. Arch. Neurol 65, 184–189. - PubMed
-
- Nakamura T & Lipton SA (2009). Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 14, 455–468. - PubMed
-
- Sengupta U, Guerrero-Muñoz MJ, Castillo-Carranza DL, Lasagna-Reeves CA, Gerson JE, Paulucci-Holthauzen AA, Krishnamurthy S, Farhed M, Jackson GR & Kayed R (2015). Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol. psychiatry 78, 672–683. - PubMed
-
- Galvin JE, Lee VM-Y & Trojanowski JQ (2001). Synucleinopathies: clinical and pathological implications. Arch. Neurol 58, 186–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

