Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis
- PMID: 33771834
- PMCID: PMC8006837
- DOI: 10.1136/rmdopen-2020-001228
Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis
Abstract
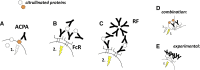
Epidemiological findings suggest a potential role for anti-citrullinated protein antibodies (ACPAs) in rheumatoid arthritis (RA) pathogenesis. ACPA-positive RA is associated with unique genetical and environmental risk factors, in contrast to seronegative RA. ACPA-positive healthy individuals are at risk of developing RA and can develop joint pain and bone loss already before disease onset. ACPA injection triggered bone loss and pain-like behaviour in mice and, in the presence of additional arthritis inducers, exacerbated joint inflammation. In cell culture experiments, ACPAs could bind to and modulate a variety of cellular targets, such as macrophages, osteoclasts, synovial fibroblasts, neutrophil granulocytes, mast cells, dendritic cells and platelets, further underlying a potential role for these autoantibodies in triggering pathogenic pathways and providing clues for their mechanisms of action. Patient-derived ACPA clones have been characterised by unique cellular effects and multiple ways to act on the target cells. ACPAs might directly induce stimulatory signals by ligating key citrullinated cell surface molecules or, alternatively, act as immune complexes on Fc receptors and potentially other molecules that recognise carbohydrate moieties. On the contrary to experimentally manufactured ACPA clones, patient-derived ACPAs are highly promiscuous and cross-reactive, suggesting a simultaneous binding to a range of functionally relevant and irrelevant targets. Moreover, several ACPA clones recognise carbamylated or acetylated targets as well. These features complicate the identification and description of ACPA-induced pathogenic mechanisms. In the current review, we summarise recent data on the functional properties of patient-derived ACPAs and present mechanistic models on how these antibodies might contribute to RA pathogenesis.
Keywords: anti-citrullinated protein antibodies; arthritis; autoimmunity; rheumatoid.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Hensvold AH, Magnusson PKE, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 2015;74:375–80. 10.1136/annrheumdis-2013-203947 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
