Disability Outcomes in the N-MOmentum Trial of Inebilizumab in Neuromyelitis Optica Spectrum Disorder
- PMID: 33771837
- PMCID: PMC8054974
- DOI: 10.1212/NXI.0000000000000978
Disability Outcomes in the N-MOmentum Trial of Inebilizumab in Neuromyelitis Optica Spectrum Disorder
Abstract
Objective: To assess treatment effects on Expanded Disability Status Scale (EDSS) score worsening and modified Rankin Scale (mRS) scores in the N-MOmentum trial of inebilizumab, a humanized anti-CD19 monoclonal antibody, in participants with neuromyelitis optica spectrum disorder (NMOSD).
Methods: Adults (N = 230) with aquaporin-4 immunoglobulin G-seropositive NMOSD or -seronegative neuromyelitis optica and an EDSS score ≤8 were randomized (3:1) to receive inebilizumab 300 mg or placebo on days 1 and 15. The randomized controlled period (RCP) was 28 weeks or until adjudicated attack, with an option to enter the inebilizumab open-label period. Three-month EDSS-confirmed disability progression (CDP) was assessed using a Cox proportional hazard model. The effect of baseline subgroups on disability was assessed by interaction tests. mRS scores from the RCP were analyzed by the Wilcoxon-Mann-Whitney odds approach.
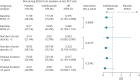
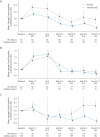
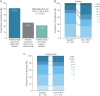
Results: Compared with placebo, inebilizumab reduced the risk of 3-month CDP (hazard ratio [HR]: 0.375; 95% CI: 0.148-0.952; p = 0.0390). Baseline disability, prestudy attack frequency, and disease duration did not affect the treatment effect observed with inebilizumab (HRs: 0.213-0.503; interaction tests: all p > 0.05, indicating no effect of baseline covariates on outcome). Mean EDSS scores improved with longer-term treatment. Inebilizumab-treated participants were more likely to have a favorable mRS outcome at the end of the RCP (OR: 1.663; 95% CI: 1.195-2.385; p = 0.0023).
Conclusions: Disability outcomes were more favorable with inebilizumab vs placebo in participants with NMOSD.
Classification of evidence: This study provides Class II evidence that for patients with NMOSD, inebilizumab reduces the risk of worsening disability. N-MOmentum is registered at ClinicalTrials.gov: NCT02200770.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. - PubMed
-
- Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–396. - PubMed
-
- Jeong IH, Choi JY, Kim SH, et al. . Normal-appearing white matter demyelination in neuromyelitis optica spectrum disorder. Eur J Neurol 2017;24:652–658. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
