Association of APOE Genotype With Heterogeneity of Cognitive Decline Rate in Alzheimer Disease
- PMID: 33771840
- PMCID: PMC8166439
- DOI: 10.1212/WNL.0000000000011883
Association of APOE Genotype With Heterogeneity of Cognitive Decline Rate in Alzheimer Disease
Abstract
Objective: To test the hypothesis that the APOE genotype is a significant driver of heterogeneity in Alzheimer disease (AD) clinical progression, which could have important implications for clinical trial design and interpretation.
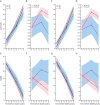
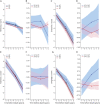
Methods: We applied novel reverse-time longitudinal models to analyze the trajectories of Clinical Dementia Rating Sum of Boxes (CDR-SOB) and Mini-Mental State Examination (MMSE) scores-2 common outcome measures in AD clinical trials-in 1,102 autopsy-proven AD cases (moderate/frequent neuritic plaques and Braak tangle stage III or greater) from the National Alzheimer's Coordinating Center Neuropathology database resembling participants with mild to moderate AD in therapeutic clinical trials.
Results: APOE ε4 carriers exhibited ≈1.5 times faster CDR-SOB increase than APOE ε3/ε3 carriers (2.12 points per year vs 1.44 points per year) and ≈1.3 times faster increase than APOE ε2 carriers (1.65 points per year), whereas APOE ε2 vs APOE ε3/ε3 difference was not statistically significant. APOE ε4 carriers had ≈1.1 times faster MMSE decline than APOE ε3/ε3 carriers (-3.45 vs -3.03 points per year) and ≈1.4 times faster decline than APOE ε2 carriers (-2.43 points per year), whereas APOE ε2 carriers had ≈1.2 times slower decline than APOE ε3/ε3 carriers (-2.43 vs -3.03 points per year). These findings remained largely unchanged after controlling for the effect of AD neuropathologic changes on the rate of cognitive decline and for the presence and severity of comorbid pathologies.
Conclusion: Compared to the APOE ε3/ε3 reference genotype, the APOE ε2 and ε4 alleles have opposite (slowing and accelerating, respectively) effects on the rate of cognitive decline, which are clinically relevant and largely independent of the differential APOE allele effects on AD and comorbid pathologies. Thus, APOE genotype contributes to the heterogeneity in rate of clinical progression in AD.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923. - PubMed
-
- Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994;7:180–184. - PubMed
-
- Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology 2003;60:246–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG049638/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 NS094610/NS/NINDS NIH HHS/United States
- R21 AG053695/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R25 NS065743/NS/NINDS NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- K08 AG064039/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
