Targeting Alzheimer's disease with multimodal polypeptide-based nanoconjugates
- PMID: 33771874
- PMCID: PMC7997513
- DOI: 10.1126/sciadv.abf9180
Targeting Alzheimer's disease with multimodal polypeptide-based nanoconjugates
Abstract
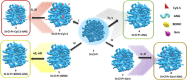
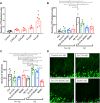
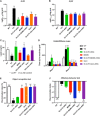
Alzheimer's disease (AD), the most prevalent form of dementia, remains incurable mainly due to our failings in the search for effective pharmacological strategies. Here, we describe the development of targeted multimodal polypeptide-based nanoconjugates as potential AD treatments. Treatment with polypeptide nanoconjugates bearing propargylamine moieties and bisdemethoxycurcumin or genistein afforded neuroprotection and displayed neurotrophic effects, as evidenced by an increase in dendritic density of pyramidal neurons in organotypic hippocampal culture. The additional conjugation of the Angiopep-2 targeting moiety enhanced nanoconjugate passage through the blood-brain barrier and modulated brain distribution with nanoconjugate accumulation in neurogenic areas, including the olfactory bulb. Nanoconjugate treatment effectively reduced neurotoxic β amyloid aggregate levels and rescued impairments to olfactory memory and object recognition in APP/PS1 transgenic AD model mice. Overall, this study provides a description of a targeted multimodal polyglutamate-based nanoconjugate with neuroprotective and neurotrophic potential for AD treatment.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


Similar articles
-
An early dysregulation of FAK and MEK/ERK signaling pathways precedes the β-amyloid deposition in the olfactory bulb of APP/PS1 mouse model of Alzheimer's disease.J Proteomics. 2016 Oct 4;148:149-58. doi: 10.1016/j.jprot.2016.07.032. Epub 2016 Aug 3. J Proteomics. 2016. PMID: 27498392
-
β-Amyloid targeting nanodrug for neuron-specific delivery of nucleic acids in Alzheimer's disease mouse models.J Control Release. 2023 Sep;361:636-658. doi: 10.1016/j.jconrel.2023.08.001. Epub 2023 Aug 17. J Control Release. 2023. PMID: 37544515
-
Colivelin Ameliorates Impairments in Cognitive Behaviors and Synaptic Plasticity in APP/PS1 Transgenic Mice.J Alzheimers Dis. 2017;59(3):1067-1078. doi: 10.3233/JAD-170307. J Alzheimers Dis. 2017. PMID: 28731445
-
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease.Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. Neuropharmacology. 2014. PMID: 23973293
-
The multimodal action of genistein in Alzheimer's and other age-related diseases.Free Radic Biol Med. 2022 Apr;183:127-137. doi: 10.1016/j.freeradbiomed.2022.03.021. Epub 2022 Mar 26. Free Radic Biol Med. 2022. PMID: 35346775 Review.
Cited by
-
Alanine and glutathione targeting of dopamine- or ibuprofen-coupled polypeptide nanocarriers increases both crossing and protective effects on a blood-brain barrier model.Fluids Barriers CNS. 2025 Feb 19;22(1):18. doi: 10.1186/s12987-025-00623-2. Fluids Barriers CNS. 2025. PMID: 39972353 Free PMC article.
-
Genistein effect on cognition in prodromal Alzheimer's disease patients. The GENIAL clinical trial.Alzheimers Res Ther. 2022 Nov 4;14(1):164. doi: 10.1186/s13195-022-01097-2. Alzheimers Res Ther. 2022. PMID: 36329553 Free PMC article. Clinical Trial.
-
Multifunctional icariin and tanshinone IIA co-delivery liposomes with potential application for Alzheimer's disease.Drug Deliv. 2022 Dec;29(1):1648-1662. doi: 10.1080/10717544.2022.2072543. Drug Deliv. 2022. PMID: 35616263 Free PMC article.
-
Endothelial cellular senescence and tau accumulation: An interplay full of opportunities?Ibrain. 2024 May 9;10(2):225-230. doi: 10.1002/ibra.12154. eCollection 2024 Summer. Ibrain. 2024. PMID: 38915948 Free PMC article.
-
"Drug-Carrier" Synergy Therapy for Amyloid-β Clearance and Inhibition of Tau Phosphorylation via Biomimetic Lipid Nanocomposite Assembly.Adv Sci (Weinh). 2022 May;9(14):e2106072. doi: 10.1002/advs.202106072. Epub 2022 Mar 20. Adv Sci (Weinh). 2022. PMID: 35307993 Free PMC article.
References
-
- Bateman R. J., Xiong C., Benzinger T. L., Fagan A. M., Goate A., Fox N. C., Marcus D. S., Cairns N. J., Xie X., Blazey T. M., Holtzman D. M., Santacruz A., Buckles V., Oliver A., Moulder K., Aisen P. S., Ghetti B., Klunk W. E., McDade E., Martins R. N., Masters C. L., Mayeux R., Ringman J. M., Rossor M. N., Schofield P. R., Sperling R. A., Salloway S., Morris J. C.; Dominantly Inherited Alzheimer Network , Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 (2012). - PMC - PubMed
-
- Alzheimer’s Statistics (2021); www.alzheimers.net/resources/alzheimers-statistics/) [accessed 13 January 2021]
-
- Burns A., Iliffe S., Alzheimer’s disease. BMJ 338, b158 (2009). - PubMed
-
- A. Onur Keskin, N. Durmaz, G. Uncu, E. Erzurumluoglu, Z. Yıldırım, N. Tuncer, D. Özbabalık Adapınar, Geriatric Medicine and Gerontology (IntechOpen, 2019).
-
- Oset-Gasque M. J., Marco-Contelles J., Alzheimer’s disease, the “one-Molecule, One-Target” paradigm, and the multitarget directed ligand approach. ACS Chem. Nerosci. 9, 401–403 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

