Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury
- PMID: 33771986
- PMCID: PMC7997909
- DOI: 10.1038/s41467-021-22137-9
Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury
Abstract
A spinal cord injury usually spares some components of the locomotor circuitry. Deep brain stimulation (DBS) of the midbrain locomotor region and epidural electrical stimulation of the lumbar spinal cord (EES) are being used to tap into this spared circuitry to enable locomotion in humans with spinal cord injury. While appealing, the potential synergy between DBS and EES remains unknown. Here, we report the synergistic facilitation of locomotion when DBS is combined with EES in a rat model of severe contusion spinal cord injury leading to leg paralysis. However, this synergy requires high amplitudes of DBS, which triggers forced locomotion associated with stress responses. To suppress these undesired responses, we link DBS to the intention to walk, decoded from cortical activity using a robust, rapidly calibrated unsupervised learning algorithm. This contingency amplifies the supraspinal descending command while empowering the rats into volitional walking. However, the resulting improvements may not outweigh the complex technological framework necessary to establish viable therapeutic conditions.
Conflict of interest statement
The authors declare the following competing interests: M.B., G.C., and S.M. hold various patents in partial relation with the present work. G.C., S.M. and are founders and shareholders of Onward, a company with partial relationships with the presented work. All other authors declare no competing interest.
Figures
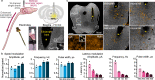

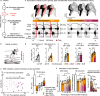



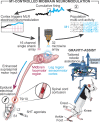

References
-
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 1993;59:75–89. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

