Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis
- PMID: 33772024
- PMCID: PMC7998027
- DOI: 10.1038/s41467-021-22272-3
Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis
Abstract
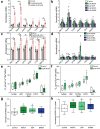
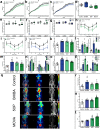
Brown and beige adipose tissue are emerging as distinct endocrine organs. These tissues are functionally associated with skeletal muscle, adipose tissue metabolism and systemic energy expenditure, suggesting an interorgan signaling network. Using metabolomics, we identify 3-methyl-2-oxovaleric acid, 5-oxoproline, and β-hydroxyisobutyric acid as small molecule metabokines synthesized in browning adipocytes and secreted via monocarboxylate transporters. 3-methyl-2-oxovaleric acid, 5-oxoproline and β-hydroxyisobutyric acid induce a brown adipocyte-specific phenotype in white adipocytes and mitochondrial oxidative energy metabolism in skeletal myocytes both in vitro and in vivo. 3-methyl-2-oxovaleric acid and 5-oxoproline signal through cAMP-PKA-p38 MAPK and β-hydroxyisobutyric acid via mTOR. In humans, plasma and adipose tissue 3-methyl-2-oxovaleric acid, 5-oxoproline and β-hydroxyisobutyric acid concentrations correlate with markers of adipose browning and inversely associate with body mass index. These metabolites reduce adiposity, increase energy expenditure and improve glucose and insulin homeostasis in mouse models of obesity and diabetes. Our findings identify beige adipose-brown adipose-muscle physiological metabokine crosstalk.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Grants and funding
- MC_PC_13030/MRC_/Medical Research Council/United Kingdom
- BB/R013500/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 16/0005382/DUK_/Diabetes UK/United Kingdom
- BB/H013539/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/R014086/1/MRC_/Medical Research Council/United Kingdom
- MC_UP_A090_1006/MRC_/Medical Research Council/United Kingdom
- MC_UU_12012/2/MRC_/Medical Research Council/United Kingdom
- MR/P01836X/1/MRC_/Medical Research Council/United Kingdom
- G0802051/MRC_/Medical Research Council/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- G0400192/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/2/MRC_/Medical Research Council/United Kingdom
- MR/P011705/1/MRC_/Medical Research Council/United Kingdom
- RG/12/13/29853/BHF_/British Heart Foundation/United Kingdom
- RG/18/7/33636/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

