MicroRNA-mediated regulation of glucose and lipid metabolism
- PMID: 33772227
- PMCID: PMC8853826
- DOI: 10.1038/s41580-021-00354-w
MicroRNA-mediated regulation of glucose and lipid metabolism
Abstract
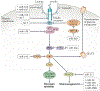
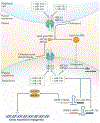
In animals, systemic control of metabolism is conducted by metabolic tissues and relies on the regulated circulation of a plethora of molecules, such as hormones and lipoprotein complexes. MicroRNAs (miRNAs) are a family of post-transcriptional gene repressors that are present throughout the animal kingdom and have been widely associated with the regulation of gene expression in various contexts, including virtually all aspects of systemic control of metabolism. Here we focus on glucose and lipid metabolism and review current knowledge of the role of miRNAs in their systemic regulation. We survey miRNA-mediated regulation of healthy metabolism as well as the contribution of miRNAs to metabolic dysfunction in disease, particularly diabetes, obesity and liver disease. Although most miRNAs act on the tissue they are produced in, it is now well established that miRNAs can also circulate in bodily fluids, including their intercellular transport by extracellular vesicles, and we discuss the role of such extracellular miRNAs in systemic metabolic control and as potential biomarkers of metabolic status and metabolic disease.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

