Tissue regulatory T cells: regulatory chameleons
- PMID: 33772242
- PMCID: PMC8403160
- DOI: 10.1038/s41577-021-00519-w
Tissue regulatory T cells: regulatory chameleons
Abstract
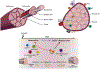
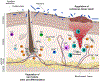
The FOXP3+CD4+ regulatory T (Treg) cells located in non-lymphoid tissues differ in phenotype and function from their lymphoid organ counterparts. Tissue Treg cells have distinct transcriptomes, T cell receptor repertoires and growth and survival factor dependencies that arm them to survive and operate in their home tissue. Their functions extend beyond immune surveillance to tissue homeostasis, including regulation of local and systemic metabolism, promotion of tissue repair and regeneration, and control of the proliferation, differentiation and fate of non-lymphoid cell progenitors. Treg cells in diverse tissues share a common FOXP3+CD4+ precursor located within lymphoid organs. This precursor undergoes definitive specialization once in the home tissue, following a multilayered array of common and tissue-distinct transcriptional programmes. Our deepening knowledge of tissue Treg cell biology will inform ongoing attempts to harness Treg cells for precision immunotherapeutics.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests. DM is a co-founder of TRex Bio and a consultant for Third Rock Ventures and Pandion Therapeutics.
Figures
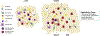

References
-
- Fontenot JD, Gavin MA, & Rudensky AY Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol 4, 330–336 (2003). - PubMed
-
- Hori S, Nomura T, & Sakaguchi S Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003). - PubMed
-
- Khattri R, Cox T, Yasayko SA, & Ramsdell F An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol 4, 337–342 (2003). - PubMed
-
- Ait-Oufella H et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med 12, 178–180 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

