New Insights into the Evolution of the Electron Transfer from Cytochrome f to Photosystem I in the Green and Red Branches of Photosynthetic Eukaryotes
- PMID: 33772595
- PMCID: PMC8557733
- DOI: 10.1093/pcp/pcab044
New Insights into the Evolution of the Electron Transfer from Cytochrome f to Photosystem I in the Green and Red Branches of Photosynthetic Eukaryotes
Abstract
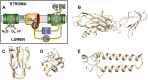
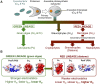
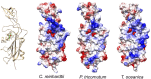
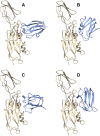
In cyanobacteria and most green algae of the eukaryotic green lineage, the copper-protein plastocyanin (Pc) alternatively replaces the heme-protein cytochrome c6 (Cc6) as the soluble electron carrier from cytochrome f (Cf) to photosystem I (PSI). The functional and structural equivalence of 'green' Pc and Cc6 has been well established, representing an example of convergent evolution of two unrelated proteins. However, plants only produce Pc, despite having evolved from green algae. On the other hand, Cc6 is the only soluble donor available in most species of the red lineage of photosynthetic organisms, which includes, among others, red algae and diatoms. Interestingly, Pc genes have been identified in oceanic diatoms, probably acquired by horizontal gene transfer from green algae. However, the mechanisms that regulate the expression of a functional Pc in diatoms are still unclear. In the green eukaryotic lineage, the transfer of electrons from Cf to PSI has been characterized in depth. The conclusion is that in the green lineage, this process involves strong electrostatic interactions between partners, which ensure a high affinity and an efficient electron transfer (ET) at the cost of limiting the turnover of the process. In the red lineage, recent kinetic and structural modeling data suggest a different strategy, based on weaker electrostatic interactions between partners, with lower affinity and less efficient ET, but favoring instead the protein exchange and the turnover of the process. Finally, in diatoms the interaction of the acquired green-type Pc with both Cf and PSI may not yet be optimized.
Keywords: Cytochrome c6; Cytochrome f; Electron transfer; Photosynthetic green and red lineages; Photosystem I; Plastocyanin.
© The Author(s) 2021. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures


References
-
- Abagyan R., Lee W.H., Raush E., Budagyan L., Totrov M., Sundstrom M., et al. (2006) Disseminating structural genomics data to the public: from a data dump to an animated story. Trends Biochem. Sci. 31: 76–78. - PubMed
-
- Akazaki H., Kawai F., Hosokawa M., Hama T., Chida H., Hirano T., et al. (2009) Crystallization and structural analysis of cytochrome c6 from the diatom Phaeodactylum tricornutum at 1.5 Å resolution. Biosci. Biotechnol. Biochem. 73: 189–191. - PubMed
-
- Antoshvili M., Caspy I., Hippler M., Nelson N. (2019) Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynth. Res. 139: 499–508. - PubMed
-
- Bendall D.S., Howe C.J. (2016) The interaction between cytochrome f and plastocyanin or cytochrome c6. InCytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling. Advances in Photosynthesis and Respiration 41. Edited by Cramer W.A. and Kallas T. pp. 631–655. Springer, Dordrecht.
-
- Bernal-Bayard P., Molina-Heredia F.P., Hervás M., Navarro J.A. (2013) Photosystem I reduction in diatoms: as complex as the green lineage systems but less efficient. Biochemistry 52: 8687–8695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

