The pharmacokinetics of pamiparib in the presence of a strong CYP3A inhibitor (itraconazole) and strong CYP3A inducer (rifampin) in patients with solid tumors: an open-label, parallel-group phase 1 study
- PMID: 33772633
- PMCID: PMC8149352
- DOI: 10.1007/s00280-021-04253-x
The pharmacokinetics of pamiparib in the presence of a strong CYP3A inhibitor (itraconazole) and strong CYP3A inducer (rifampin) in patients with solid tumors: an open-label, parallel-group phase 1 study
Abstract
Purpose: Pamiparib is an investigational, selective, oral poly(ADP-ribose) polymerase 1/2 (PARP1/2) inhibitor that has demonstrated PARP-DNA complex trapping and CNS penetration in preclinical models, as well as preliminary anti-tumor activity in early-phase clinical studies. We investigated whether the single-dose pharmacokinetic (PK) profile of pamiparib is altered by coadministration of a strong CYP3A inducer (rifampin) or a strong CYP3A inhibitor (itraconazole) in patients with solid tumors.
Methods: In this open-label, phase 1 study, adults with advanced solid tumors received either oral pamiparib 60 mg (days 1 and 10) and once-daily oral rifampin 600 mg (days 3-11) or oral pamiparib 20 mg (days 1 and 7) and once-daily oral itraconazole 200 mg (days 3-8). Primary endpoints included pamiparib maximum observed concentration (Cmax), and area under the plasma concentration-time curve from zero to last quantifiable concentration (AUC0-tlast) and infinity (AUC0-inf). Secondary endpoints included safety and tolerability.
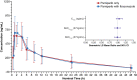
Results: Rifampin coadministration did not affect pamiparib Cmax (geometric least-squares [GLS] mean ratio 0.94; 90% confidence interval 0.83-1.06), but reduced its AUC0-tlast (0.62 [0.54-0.70]) and AUC0-inf (0.57 [0.48-0.69]). Itraconazole coadministration did not affect pamiparib Cmax (1.05 [0.95-1.15]), AUC0-tlast (0.99 [0.91-1.09]), or AUC0-inf (0.99 [0.90-1.09]). There were no serious treatment-related adverse events.
Conclusions: Pamiparib plasma exposure was reduced 38-43% with rifampin coadministration but was unaffected by itraconazole coadministration. Pamiparib dose modifications are not considered necessary when coadministered with CYP3A inhibitors. Clinical safety and efficacy data will be used with these results to recommend dose modifications when pamiparib is coadministered with CYP3A inducers.
Keywords: Anticancer agents; Anticancer drugs; CYP3A; Clinical pharmacokinetics; Phase I, II and III trials; Solid tumors.
Conflict of interest statement
Song Mu, Claudia Andreu-Vieyra, Chester Lin, and Srikumar Sahasranaman are employees with stock grant or options in BeiGene. Anna Skrzypczyk-Ostaszewicz, Viera Skarbova, Marina Maglakelidze, and Iurie Bulat have nothing to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

