Patient-Specific Modelling of Blood Coagulation
- PMID: 33772645
- PMCID: PMC7998098
- DOI: 10.1007/s11538-021-00890-8
Patient-Specific Modelling of Blood Coagulation
Abstract
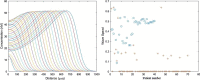
Blood coagulation represents one of the most studied processes in biomedical modelling. However, clinical applications of this modelling remain limited because of the complexity of this process and because of large inter-patient variation of the concentrations of blood factors, kinetic constants and physiological conditions. Determination of some of these patients-specific parameters is experimentally possible, but it would be related to excessive time and material costs impossible in clinical practice. We propose in this work a methodological approach to patient-specific modelling of blood coagulation. It begins with conventional thrombin generation tests allowing the determination of parameters of a reduced kinetic model. Next, this model is used to study spatial distributions of blood factors and blood coagulation in flow, and to evaluate the results of medical treatment of blood coagulation disorders.
Keywords: Blood coagulation; Blood flow; Reaction–diffusion waves; Thrombin generation curves; Treatment.
Figures












References
-
- Anand M, Rajagopal K, Rajagopal K. A model incorporating some of the mechanical and biochemical factors underlying clot formation and dissolution in flowing blood. J Theor Med. 2003;5(3–4):183–218.
-
- Andreeva AA, Anand M, Lobanov AI, Nikolaev AV, Panteleev MA, Susree M (2018) Mathematical modelling of platelet rich plasma clotting. Pointwise unified model. Russ J Numer Anal Math Modell 33(5):265–276
-
- Beavers G, Joseph D. Boundary conditions at a naturally permeable wall. J Fluid Mech. 1967;30(01):197–207.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

