A single subcutaneous or intranasal immunization with adenovirus-based SARS-CoV-2 vaccine induces robust humoral and cellular immune responses in mice
- PMID: 33772778
- PMCID: PMC8250272
- DOI: 10.1002/eji.202149167
A single subcutaneous or intranasal immunization with adenovirus-based SARS-CoV-2 vaccine induces robust humoral and cellular immune responses in mice
Abstract
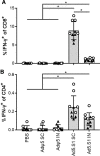
Optimal vaccines are needed for sustained suppression of SARS-CoV-2 and other novel coronaviruses. Here, we developed a recombinant type 5 adenovirus vector encoding the gene for the SARS-CoV-2 S1 subunit antigen (Ad5.SARS-CoV-2-S1) for COVID-19 immunization and evaluated its immunogenicity in mice. A single immunization with Ad5.SARS-CoV-2-S1 via S.C. injection or I.N delivery induced robust antibody and cellular immune responses. Vaccination elicited significant S1-specific IgG, IgG1, and IgG2a endpoint titers as early as 2 weeks, and the induced antibodies were long lasting. I.N. and S.C. administration of Ad5.SARS-CoV-2-S1 produced S1-specific GC B cells in cervical and axillary LNs, respectively. Moreover, I.N. and S.C. immunization evoked significantly greater antigen-specific T-cell responses compared to unimmunized control groups with indications that S.C. injection was more effective than I.N. delivery in eliciting cellular immune responses. Mice vaccinated by either route demonstrated significantly increased virus-specific neutralization antibodies on weeks 8 and 12 compared to control groups, as well as BM antibody forming cells (AFC), indicative of long-term immunity. Thus, this Ad5-vectored SARS-CoV-2 vaccine candidate showed promising immunogenicity following delivery to mice by S.C. and I.N. routes of administration, supporting the further development of Ad-based vaccines against COVID-19 and other infectious diseases for sustainable global immunization programs.
Keywords: Adenovirus; COVID-19; Infectious diseases; Recombinant DNA vaccines; SARS-CoV-2.
© 2021 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
Conceptualization: EK, MJS, LDF, AG; Data Curation: EK, FJW, SCB, SMJ, EP, IC, FB; Formal Analysis: EK, FJW, SCB, Investigation: EK, FJW, SCB, MSK, SH, GE, TWK, CDC, SMJ, LJC, NMW, NMAO, BLH, EP, IC, FB; Methodology: EK, FJW, MJS, LDF, AG; Resources: MJS, LDF, AG; Visualization: EK, FJW, SCB; Original Draft Preparation: EK, FJW, SCB; Review and Editing: MSK, SH, GE, TWK, CDC, SMJ, LJC, NMW, NMAO, BLH, EP, IC, FB, EK, MJS, LDF, AG.
The authors declare no commercial or financial conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

