Pharmacological and neurosurgical interventions for individuals with cerebral palsy and dystonia: a systematic review update and meta-analysis
- PMID: 33772789
- PMCID: PMC8451898
- DOI: 10.1111/dmcn.14874
Pharmacological and neurosurgical interventions for individuals with cerebral palsy and dystonia: a systematic review update and meta-analysis
Abstract
Aim: To update a systematic review of evidence published up to December 2015 for pharmacological/neurosurgical interventions among individuals with cerebral palsy (CP) and dystonia.
Method: Searches were updated (January 2016 to May 2020) for oral baclofen, trihexyphenidyl, benzodiazepines, clonidine, gabapentin, levodopa, botulinum neurotoxin (BoNT), intrathecal baclofen (ITB), and deep brain stimulation (DBS), and from database inception for medical cannabis. Eligible studies included at least five individuals with CP and dystonia and reported on dystonia, goal achievement, motor function, pain/comfort, ease of caregiving, quality of life (QoL), or adverse events. Evidence certainty was evaluated using GRADE.
Results: Nineteen new studies met inclusion criteria (two trihexyphenidyl, one clonidine, two BoNT, nine ITB, six DBS), giving a total of 46 studies (four randomized, 42 non-randomized) comprising 915 participants when combined with those from the original systematic review. Very low certainty evidence supported improved dystonia (clonidine, ITB, DBS) and goal achievement (clonidine, BoNT, ITB, DBS). Low to very low certainty evidence supported improved motor function (DBS), pain/comfort (clonidine, BoNT, ITB, DBS), ease of caregiving (clonidine, BoNT, ITB), and QoL (ITB, DBS). Trihexyphenidyl, clonidine, BoNT, ITB, and DBS may increase adverse events. No studies were identified for benzodiazepines, gabapentin, oral baclofen, and medical cannabis.
Interpretation: Evidence evaluating the use of pharmacological and neurosurgical management options for individuals with CP and dystonia is limited to between low and very low certainty. What this paper adds Meta-analysis suggests that intrathecal baclofen (ITB) and deep brain stimulation (DBS) may improve dystonia and pain. Meta-analysis suggests that DBS may improve motor function. Clonidine, botulinum neurotoxin, ITB, and DBS may improve achievement of individualized goals. ITB and DBS may improve quality of life. No direct evidence is available for oral baclofen, benzodiazepines, gabapentin, or medical cannabis.
Objetivo: Actualizar una revisión sistemática sobre evidencia publicada hasta Diciembre del 2015 para intervenciones farmacológicas y neuroquirúrgicas entre individuos con parálisis cerebral (PC) y distonía. MÉTODO: Se actualizaron las búsquedas (desde Enero 2016 hasta Mayo del 2020) para baclofeno oral, trihexifenidilo, benzodiacepinas, clonidina, gabapentina, levodopa, neurotoxina botulinica (BoNT), baclofeno intratecal (ITB), y estimulación cerebral profunda (DBS), y desde el inicio de la base de datos para el cannabis medicinal. Los estudios elegibles incluyeron al menos 5 individuos con PC y distonía e informaron sobre distonía, logro de metas, función motora, dolor/comorbilidad, facilidad para brindar cuidados, calidad de vida (QoL) o eventos adversos. La certeza de la evidencia se evaluó mediante GRADE.
Resultados: Diez y nueve estudios reunieron los criterios de inclusión (2 trihexifenidilo, uno clonidina, 2 BoNT, 9 ITB, 6 DBS). Cuando se combinan con los de la revisión sistemática original, dan un total de 46 estudios (cuatro aleatorios, 42 no aleatorios) que comprenden 915 participantes. Evidencia de certeza muy baja apoyó la mejoría de la distonía (clonidina, BoNT, ITB, DBS). La certeza baja a una muy baja apoyó una mejor función motora (DBS), dolor/comorbilidad (clonidina, BoNT, ITB, DBS), facilidad de cuidado (clonidina, BoNT, ITB) y CdV (ITB, DBS). Trihexifenidilo, clonidina, BoNT, ITB y DBS pueden aumentar los eventos adversos. No se identificaron estudios para benzodiacepinas, gabapentina, baclofeno oral, y cannabis medicinal. INTERPRETACIÓN: La evidencia que evalúa el uso de opciones de manejo farmacológico y neuroquirúrgico para personas con parálisis cerebral y distonía se limita a evidencia entre baja y muy baja certeza.
Objetivo: Atualizar uma revisão sistemática da evidência publicada até dezembro de 2015 para intervenções farmacológicas/neurocirúrgicas entre indivíduos com paralisia cerebral (PC) e distonia. MÉTODO: As buscas foram atualizadas (Janeiro 2016 a Maio 2020) quanto a baclofeno oral, triexifenidil, benzodiazepínicos, clonidina, gabapentina, levodopa, neurotoxina botulínica (NTBo), baclofeno intratecal (BIT), e estimulação cerebral profunda (ECB), e desde o início da base de dados para cannabis medicinal. Estudos elegíveis incluíram ao menos cinco indivíduos com PC e dystonia, e reportaram os objetivos atingidos, função motora, dor/conforto, facilidade do cuidado, qualidade de vida (QV), ou efeitos adversos. A certeza da evidência foi avaliada usando GRADE.
Resultados: Dezenove novos estudos atenderam aos critérios de inclusão (dois com triexifenidil 1 com clonidina, dois com NTBo, nove com BIT e seis com ECB), dando um total de 46 estudos (quatro randomizados, 42 não randomizados) compreendendo 915 participantes quando combinados com aqueles da revisão sistemática original. Evidência com certeza muito baixa suporta a melhora da distonia (clonidina, BIT, ECB) e atingimento de objetivos (clonidina, NTBo, BIT, ECB). Evidência com certeza baixa a muito baixa apóia melhora da função motora (ECB), dor/conforto (clonidina, NTBo, BIT, ECB), facilidade de cuidado (clonidina, NTBo, BIT), e QV (BIT, ECB). Triexifenidil, clonidina, NTBo, BIT, e ECB podem aumentar efeitos adversos. Não foram identificados estudos com benzodiazepínicos, gabapentina, baclofeno oral, e cannabis medicinal. INTERPRETAÇÃO: A evidência avaliando o uso de opções de manejo farmacológico e cirúrgico para indivíduos com PC e distonia é limitada a certeza baixa e muito baixa.
© 2021 The Authors. Developmental Medicine & Child Neurology published by John Wiley & Sons Ltd on behalf of Mac Keith Press.
Figures

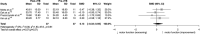




References
-
- Monbaliu E, de Cock P, Ortibus E, Heyrman L, Klingels K, Feys H. Clinical patterns of dystonia and choreoathetosis in participants with dyskinetic cerebral palsy. Dev Med Child Neurol 2016; 58: 138–44. - PubMed
-
- Graham D, Paget SP, Wimalasundera N. Current thinking in the health care management of children with cerebral palsy. Med J Aust 2019; 210: 129–35. - PubMed
-
- Harvey A, Reddihough D, Scheinberg A, Williams K. Oral medication prescription practices of tertiary‐based specialists for dystonia in children with cerebral palsy. J Paediatr Child Health 2018; 54: 401–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

