CytoBas: Precision component-resolved diagnostics for allergy using flow cytometric staining of basophils with recombinant allergen tetramers
- PMID: 33772805
- PMCID: PMC8518718
- DOI: 10.1111/all.14832
CytoBas: Precision component-resolved diagnostics for allergy using flow cytometric staining of basophils with recombinant allergen tetramers
Abstract
Background: Diagnostic tests for allergy rely on detecting allergen-specific IgE. Component-resolved diagnostics incorporate multiple defined allergen components to improve the quality of diagnosis and patient care.
Objective: To develop a new approach for determining sensitization to specific allergen components that utilizes fluorescent protein tetramers for direct staining of IgE on blood basophils by flow cytometry.
Methods: Recombinant forms of Lol p 1 and Lol p 5 proteins from ryegrass pollen (RGP) and Api m 1 from honeybee venom (BV) were produced, biotinylated, and tetramerized with streptavidin-fluorochrome conjugates. Blood samples from 50 RGP-allergic, 41 BV-allergic, and 26 controls were incubated with fluorescent protein tetramers for flow cytometric evaluation of basophil allergen binding and activation.
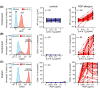
Results: Allergen tetramers bound to and activated basophils from relevant allergic patients but not controls. Direct fluorescence staining of Api m 1 and Lol p 1 tetramers had greater positive predictive values than basophil activation for BV and RGP allergy, respectively, as defined with receiver operator characteristics (ROC) curves. Staining intensities of allergen tetramers correlated with allergen-specific IgE levels in serum. Inclusion of multiple allergens coupled with distinct fluorochromes in a single-tube assay enabled rapid detection of sensitization to both Lol p 1 and Lol p 5 in RGP-allergic patients and discriminated between controls, BV-allergic, and RGP-allergic patients.
Conclusion: Our novel flow cytometric assay, termed CytoBas, enables rapid and reliable detection of clinically relevant allergic sensitization. The intensity of fluorescent allergen tetramer staining of basophils has a high positive predictive value for disease, and the assay can be multiplexed for a component-resolved and differential diagnostic test for allergy.
Keywords: allergen tetramers; basophils; component-resolved diagnostics; flow cytometry; surface IgE.
© 2021 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
MCvZ, ROH, and PMH are inventors on a patent application related to this work. All the other authors declare that they have no relevant conflicts of interest.
Figures





References
-
- van Zelm MC, McKenzie CI, Varese N, Rolland JM, O'Hehir RE. Recent developments and highlights in immune monitoring of allergen immunotherapy. Allergy 2019;74(12):2342‐2354. - PubMed
-
- Carnés J, Iraola V, Cho SH, Esch RE. Mite allergen extracts and clinical practice. Ann Allergy Asthma Immunol 2017;118(3):249‐256. - PubMed
-
- Codina R, Lockey RF. Pollen used to produce allergen extracts. Ann Allergy Asthma Immunol 2017;118(2):148‐153. - PubMed
-
- Frick M, Fischer J, Helbling A, et al. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol Pract. 2016;138(6):1663‐1671.e9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

