Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson's Disease
- PMID: 33772855
- PMCID: PMC8251848
- DOI: 10.1002/ana.26073
Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson's Disease
Abstract
Objective: The aim was to demonstrate that continuous s.c. infusion of a soluble levodopa (LD)/carbidopa (CD) phosphate prodrug combination effectively delivers stable LD exposure via a minimally invasive and convenient mode and has the potential to treat Parkinson's disease (PD) patients who are not well controlled on oral medication.
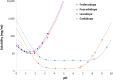
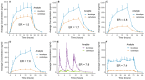
Methods: Foslevodopa and foscarbidopa were prepared and the equilibrium solubility and chemical stability examined in aqueous media with different values of pH. Solutions of foslevodopa/foscarbidopa (ratios ranging from 4:1 to 20:1) were prepared by dissolving pH-adjusted lyophilized materials in water and infused s.c. in healthy volunteers for ≤72 hours. Frequent blood samples were collected to measure LD and CD exposure, and safety was monitored throughout the study.
Results: Foslevodopa/foscarbidopa (ABBV-951) demonstrates high water solubility and excellent chemical stability near physiological pH, enabling continuous s.c. infusion therapy. After s.c. infusion, a stable LD pharmacokinetic (PK) profile was maintained for ≤72 hours, and the infusion was well tolerated.
Interpretation: Preparation of foslevodopa and foscarbidopa enables preclinical and clinical PK, safety, and tolerability studies in support of their advancement for the treatment of PD. In phase 1 clinical trials, foslevodopa/foscarbidopa demonstrates consistent and stable LD plasma exposure, supporting further studies of this treatment as a potentially transformational option for those suffering from PD. ANN NEUROL 2021;90:52-61.
© 2021 AbbVie Inc. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
M.R., E.A.V., E.M.M., X.L., G.G.Z.Z., P.T.M., D.S., R.A.C., B.P.E., W.L., M.F.F., and P.R.K.: AbbVie employees and may hold stock or options. F.J.: AbbVie employee at the time this work was conducted and may hold stock or options.
Figures


References
-
- Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896–912. - PubMed
-
- Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 2002;14:223–236. - PubMed
-
- Parent M, Parent A. Substantia nigra and Parkinson's disease: a brief history of their long and intimate relationship. Can J Neurol Sci 2010;37:313–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

