Immunoglobin G/total antibody testing for SARS-CoV-2: A prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests
- PMID: 33773276
- PMCID: PMC7914028
- DOI: 10.1016/j.ejca.2021.02.024
Immunoglobin G/total antibody testing for SARS-CoV-2: A prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests
Abstract
Background: Coronavirus disease (COVID-19) is interfering heavily with the screening, diagnosis and treatment of cancer patients. Better knowledge of the seroprevalence and immune response after Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in this population is important to manage them safely during the pandemic.
Methods: 922 cancer patients, 100 non-cancer patients and 94 health care workers (HCW) attending the Multidisciplinary Oncology Unit of Antwerp University Hospital from 24th of March 2020 till 31st of May 2020, and the Oncology Unit of AZ Maria Middelares Hospital, Ghent, from 13th of April 2020 till 31st of May 2020 participated in the study. The Alinity® (A; Abbott) and Liaison® (D; DiaSorin) commercially available assays were used to measure SARS-CoV-2 IgG, while total SARS-CoV-2 Ig was measured by Elecsys® (R; Roche).
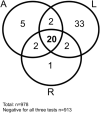
Results: In the overall study population IgG/total SARS-CoV-2 antibodies were found in respectively 32/998 (3.2%), 68/1020 (6.7%), 37/1010 (3.7%) and of individuals using the A, D or R test. Forty-six out of 618 (7.4%) persons had a positive SARS-CoV-2 polymerase chain reaction (RT-PCR) test. Seroprevalence in cancer patients (A:2.2%, D:6.2%, R:3.0%), did not significantly differ from that in non-cancer patients (A:1.1%, D:5.6%, R:0.0%), but was lower than the HCW (A:13%, D:12%, R:12%; respectively Fisher's exact test p = 0.00001, p = 0.046, p = 0.0004). A positive SARS-CoV-2 RT-PCR was found in 6.8% of the cancer patients, 2.3% of the non-cancer patients and 28.1% of the HCW (Fisher's exact test p = 0.0004). Correlation between absolute values of the different Ig tests was poor in the cancer population. Dichotomising a positive versus negative test result, the A and R test correlated well (kappa 0.82 p McNemar test = 0.344), while A and D and R and D did not (respectively kappa 0.49 and 0.57; result significantly different p McNemar test = <0.0001 for both). The rate of seroconversion (>75%) and median absolute antibody levels (A: 7.0 versus 4.7; D 74.0 versus 26.6, R: 16.34 versus 7.32; all >P Mann Whitney U test = 0.28) in cancer patients and HCW with a positive RT-PCR at least 7 days earlier did not show any differences. However, none (N = 0/4) of the patients with hematological tumours had seroconversion and absolute antibody levels remained much lower compared to patients with solid tumours (R: 0.1 versus 37.6, p 0.003; D 4.1 versus 158, p 0.008) or HCW (all p < 0.0001).
Conclusion: HCW were at high risk of being infected by SARS-CoV-2 during the first wave of the pandemic. Seroprevalence in cancer patients was low in the study period. Although Ig immune response in cancer patients with solid tumours does not differ from healthy volunteers, patients with hematological tumours have a very poor humoral immune response. This has to be taken into account in future vaccination programmes in this population. SARS-CoV-2 antibody tests have divergent results and seem to have little added value in the management of cancer patients.
Keywords: COVID-19; Cancer; Health care workers; Immunoglobulin G; SARS-CoV-2; Seroconversion.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement MP is an advisor of Remedus; none of the other authors has a conflict of interest related to this paper.
Figures

Similar articles
-
SARS-CoV-2 Antibody Testing in Health Care Workers: A Comparison of the Clinical Performance of Three Commercially Available Antibody Assays.Microbiol Spectr. 2021 Oct 31;9(2):e0039121. doi: 10.1128/Spectrum.00391-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585976 Free PMC article.
-
The reliability of SARS-CoV-2 IgG antibody testing - a pilot study in asymptomatic health care workers in a Croatian university hospital.Croat Med J. 2020 Dec 31;61(6):485-490. doi: 10.3325/cmj.2020.61.485. Croat Med J. 2020. PMID: 33410294 Free PMC article.
-
Provider Antibody Serology Study of Virus in the Emergency Room (PASSOVER) Study: Special Population COVID-19 Seroprevalence.West J Emerg Med. 2021 Apr 9;22(3):565-571. doi: 10.5811/westjem.2021.1.50058. West J Emerg Med. 2021. PMID: 34125028 Free PMC article.
-
Seroprevalence of SARS-CoV-2-specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data.Cancer Treat Rev. 2020 Nov;90:102102. doi: 10.1016/j.ctrv.2020.102102. Epub 2020 Sep 1. Cancer Treat Rev. 2020. PMID: 32947121 Free PMC article. Review.
-
Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis.J Hosp Infect. 2021 Feb;108:120-134. doi: 10.1016/j.jhin.2020.11.008. Epub 2020 Nov 16. J Hosp Infect. 2021. PMID: 33212126 Free PMC article.
Cited by
-
The impact of the SARS-COV-2 pandemic on the quality of breast cancer care in EUSOMA-certified breast centres.Eur J Cancer. 2022 Dec;177:72-79. doi: 10.1016/j.ejca.2022.09.027. Epub 2022 Oct 12. Eur J Cancer. 2022. PMID: 36332437 Free PMC article.
-
Long-term effects of the COVID-19 pandemic for patients with cancer.Qual Life Res. 2024 Oct;33(10):2845-2853. doi: 10.1007/s11136-024-03726-9. Epub 2024 Jul 3. Qual Life Res. 2024. PMID: 38961007 Free PMC article.
-
Blood Cytokine Analysis Suggests That SARS-CoV-2 Infection Results in a Sustained Tumour Promoting Environment in Cancer Patients.Cancers (Basel). 2021 Nov 15;13(22):5718. doi: 10.3390/cancers13225718. Cancers (Basel). 2021. PMID: 34830872 Free PMC article.
-
COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies.Br J Haematol. 2021 Nov;195(3):371-377. doi: 10.1111/bjh.17704. Epub 2021 Jul 16. Br J Haematol. 2021. PMID: 34272724 Free PMC article. Clinical Trial.
-
Predictive model for BNT162b2 vaccine response in cancer patients based on blood cytokines and growth factors.Front Immunol. 2022 Dec 22;13:1062136. doi: 10.3389/fimmu.2022.1062136. eCollection 2022. Front Immunol. 2022. PMID: 36618384 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

