Impaired performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests at elevated and low temperatures
- PMID: 33773413
- PMCID: PMC7962993
- DOI: 10.1016/j.jcv.2021.104796
Impaired performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests at elevated and low temperatures
Abstract
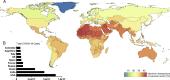
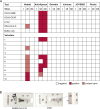
Antigen-detecting rapid diagnostic tests (Ag-RDTs) can complement molecular diagnostics for COVID-19. The recommended temperature for storage of SARS-CoV-2 Ag-RDTs ranges between 2-30 °C. In the global South, mean temperatures can exceed 30 °C. In the global North, Ag-RDTs are often used in external testing facilities at low ambient temperatures. We assessed analytical sensitivity and specificity of eleven commercially-available SARS-CoV-2 Ag-RDTs using different storage and operational temperatures, including short- or long-term storage and operation at recommended temperatures or at either 2-4 °C or at 37 °C. The limits of detection of SARS-CoV-2 Ag-RDTs under recommended conditions ranged from 1.0×106- 5.5×107 genome copies/mL of infectious SARS-CoV-2 cell culture supernatant. Despite long-term storage at recommended conditions, 10 min pre-incubation of Ag-RDTs and testing at 37 °C resulted in about ten-fold reduced sensitivity for five out of 11 SARS-CoV-2 Ag-RDTs, including both Ag-RDTs currently listed for emergency use by the World Health Organization. After 3 weeks of storage at 37 °C, eight of the 11 SARS-CoV-2 Ag-RDTs exhibited about ten-fold reduced sensitivity. Specificity of SARS-CoV-2 Ag-RDTs using cell culture supernatant from common respiratory viruses was not affected by storage and testing at 37 °C, whereas false-positive results occurred at outside temperatures of 2-4 °C for two out of six tested Ag-RDTs, again including an Ag-RDT recommended by the WHO. In summary, elevated temperatures impair sensitivity, whereas low temperatures impair specificity of SARS-CoV-2 Ag-RDTs. Consequences may include false-negative test results at clinically relevant virus concentrations compatible with transmission and false-positive results entailing unwarranted quarantine assignments. Storage and operation of SARS-CoV-2 Ag-RDTs at recommended conditions is essential for successful usage during the pandemic.
Keywords: Rapid antigen test; SARS-CoV-2; Sensitivity; Specificity; Temperature stability; Tropics; Winter.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of competing interest.
Figures


References
-
- Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Mühlemann B., Zuchowski M., Jó Lei W.K., Tscheak P., Möncke-Buchner E., Müller M.A., Krumbholz A., Drexler J.F., Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests. medRxiv. 2020 doi: 10.1101/2020.11.12.20230292:2020.11.12.20230292. - DOI - PMC - PubMed
-
- Iglὁi Z., Velzing J., van Beek J., van de Vijver D., Aron G., Ensing R., Benschop K., Han W., Boelsums T., Koopmans M., Geurtsvankessel C., Molenkamp R. Clinical evaluation of the Roche/SD Biosensor rapid antigen test with symptomatic, non-hospitalized patients in a municipal health service drive-through testing site. medRxiv. 2020 doi: 10.1101/2020.11.18.20234104:2020.11.18.20234104. - DOI
-
- Krüger L.J., Gaeddert M., Köppel L., Brümmer L.E., Gottschalk C., Miranda I.B., Schnitzler P., Kräusslich H.G., Lindner A.K., Nikolai O., Mockenhaupt F.P., Seybold J., Corman V.M., Drosten C., Pollock N.R., Cubas-Atienzar A.I., Kontogianni K., Collins A., Wright A.H., Knorr B., Welker A., de Vos M., Sacks J.A., Adams E.R., Denkinger C.M. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.10.01.20203836:2020.10.01.20203836. - DOI
-
- Lindner A.K., Nikolai O., Rohardt C., Burock S., Hülso C., Bölke A., Gertler M., Krüger L.J., Gaeddert M., Tobian F., Lainati F., Seybold J., Jones T.C., Hofmann J., Sacks J.A., Mockenhaupt F.P., Denkinger C.M. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with professional-collected anterior nasal versus nasopharyngeal swab. medRxiv. 2020 doi: 10.1101/2020.12.03.20243725:2020.12.03.20243725. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

