Early detection of amyloid load using 18F-florbetaben PET
- PMID: 33773598
- PMCID: PMC8005243
- DOI: 10.1186/s13195-021-00807-6
Early detection of amyloid load using 18F-florbetaben PET
Abstract
Background: A low amount and extent of Aβ deposition at early stages of Alzheimer's disease (AD) may limit the use of previously developed pathology-proven composite SUVR cutoffs. This study aims to characterize the population with earliest abnormal Aβ accumulation using 18F-florbetaben PET. Quantitative thresholds for the early (SUVRearly) and established (SUVRestab) Aβ deposition were developed, and the topography of early Aβ deposition was assessed. Subsequently, Aβ accumulation over time, progression from mild cognitive impairment (MCI) to AD dementia, and tau deposition were assessed in subjects with early and established Aβ deposition.
Methods: The study population consisted of 686 subjects (n = 287 (cognitively normal healthy controls), n = 166 (subjects with subjective cognitive decline (SCD)), n = 129 (subjects with MCI), and n = 101 (subjects with AD dementia)). Three categories in the Aβ-deposition continuum were defined based on the developed SUVR cutoffs: Aβ-negative subjects, subjects with early Aβ deposition ("gray zone"), and subjects with established Aβ pathology.
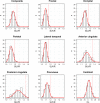
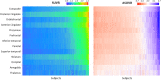
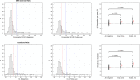
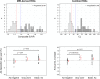
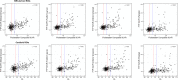
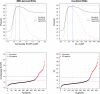
Results: SUVR using the whole cerebellum as the reference region and centiloid (CL) cutoffs for early and established amyloid pathology were 1.10 (13.5 CL) and 1.24 (35.7 CL), respectively. Cingulate cortices and precuneus, frontal, and inferior lateral temporal cortices were the regions showing the initial pathological tracer retention. Subjects in the "gray zone" or with established Aβ pathology accumulated more amyloid over time than Aβ-negative subjects. After a 4-year clinical follow-up, none of the Aβ-negative or the gray zone subjects progressed to AD dementia while 91% of the MCI subjects with established Aβ pathology progressed. Tau deposition was infrequent in those subjects without established Aβ pathology.
Conclusions: This study supports the utility of using two cutoffs for amyloid PET abnormality defining a "gray zone": a lower cutoff of 13.5 CL indicating emerging Aβ pathology and a higher cutoff of 35.7 CL where amyloid burden levels correspond to established neuropathology findings. These cutoffs define a subset of subjects characterized by pre-AD dementia levels of amyloid burden that precede other biomarkers such as tau deposition or clinical symptoms and accelerated amyloid accumulation. The determination of different amyloid loads, particularly low amyloid levels, is useful in determining who will eventually progress to dementia. Quantitation of amyloid provides a sensitive measure in these low-load cases and may help to identify a group of subjects most likely to benefit from intervention.
Trial registration: Data used in this manuscript belong to clinical trials registered in ClinicalTrials.gov ( NCT00928304 , NCT00750282 , NCT01138111 , NCT02854033 ) and EudraCT (2014-000798-38).
Keywords: Alzheimer’s disease; Amyloid-beta; Florbetaben; Mild cognitive impairment; PET; Subjective memory complainers.
Conflict of interest statement
SB, NRV, NK, AM, AP, AJ, and AS are employees of Life Molecular Imaging GmbH (formerly Piramal Imaging GmbH). SDS is an employee of Eisai Inc. and a former employee of Life Molecular Imaging Inc. (formerly Piramal Pharma Inc). HB and OS received research support, consultant honoraria, and travel expenses from Piramal Imaging GmbH. Victor L. Villemagne has received speaker’s honoraria from Piramal Imaging, GE Healthcare, Avid Pharmaceuticals, AstraZeneca, and Hoffmann-La Roche and consulting fees for Novartis, Lundbeck, Abbvie, Shanghai Green Valley Pharmaceutical Co. LTD, and Hoffmann-La Roche. Christopher C. Rowe has received research grants from Bayer Schering Pharma, Piramal Imaging, Avid Radiopharmaceuticals, Navidea, GE Healthcare, AstraZeneca, and Biogen. John Seibyl holds equity in Invicro and consulting fees from LMI, Roche, Biogen, AbVie, and Invicro. M. Boada has received research funds from the following private donors: Grifols SA, Caixabank S.A., Piramal Imaging, Araclon Biotech, Laboratorios Echevarne, Fundació Castell de Peralada, and Fundació La Pedrera and has participated in advisory boards of Araclon Biotech, Biogen, Bioibérica, Eisai, Grifols, Lilly, Merck, Nutricia, Roche, Schwabe Farma, Servier, and Kyowa Kirin.
No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Uenaka K, Nakano M, Willis BA, Friedrich S, Ferguson-Sells L, Dean RA, et al. Comparison of pharmacokinetics, pharmacodynamics, safety, and tolerability of the amyloid beta monoclonal antibody solanezumab in Japanese and white patients with mild to moderate Alzheimer disease. Clin Neuropharmacol. 2012;35(1):25–29. doi: 10.1097/WNF.0b013e31823a13d3. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

