MAML1: a coregulator that alters endometrial epithelial cell adhesive capacity
- PMID: 33773601
- PMCID: PMC8004388
- DOI: 10.1186/s40738-021-00100-y
MAML1: a coregulator that alters endometrial epithelial cell adhesive capacity
Abstract
Background: Abnormalities in endometrial receptivity has been identified as a major barrier to successful embryo implantation. Endometrial receptivity refers to the conformational and biochemical changes occurring in the endometrial epithelial layer which make it adhesive and receptive to blastocyst attachment. This takes place during the mid-secretory phase of woman's menstrual cycle and is a result of a delicate interplay between numerous hormones, cytokines and other factors. Outside of this window, the endometrium is refractory to an implanting blastocyst. It has been shown that Notch ligands and receptors are dysregulated in the endometrium of infertile women. Mastermind Like Transcriptional Coactivator 1 (MAML1) is a known coactivator of the Notch signaling pathway. This study aimed to determine the role of MAML1 in regulating endometrial receptivity.
Methods: The expression and localization of MAML1 in the fertile human endometrium (non-receptive proliferative phase versus receptive mid-secretory phase) were determined by immunohistochemistry. Ishikawa cells were used as an endometrial epithelial model to investigate the functional consequences of MAML1 knockdown on endometrial adhesive capacity to HTR8/SVneo (trophoblast cell line) spheroids. After MAML1 knockdown in Ishikawa cells, the expression of endometrial receptivity markers and Notch dependent and independent pathway members were assessed by qPCR. Two-tailed unpaired or paired student's t-test were used for statistical analysis with a significance threshold of P < 0.05.
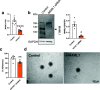
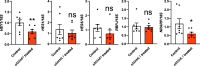
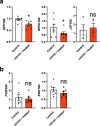
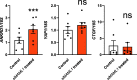
Results: MAML1 was localized in the luminal epithelium, glandular epithelium and stroma of human endometrium and the increased expression identified in the mid-secretory phase was restricted only to the luminal epithelium (P < 0.05). Functional analysis using Ishikawa cells demonstrated that knockdown of MAML1 significantly reduced epithelial adhesive capacity (P < 0.01) to HTR8/SVneo (trophoblast cell line) spheroids compared to control. MAML1 knockdown significantly affected the expression of classical receptivity markers (SPP1, DPP4) and this response was not directly via hormone receptors. The expression level of Hippo pathway target Ankyrin repeat domain-containing protein 1 (ANKRD1) was also affected after MAML1 knockdown in Ishikawa cells.
Conclusion: Our data strongly suggest that MAML1 is involved in regulating the endometrial adhesive capacity and may facilitate embryo attachment, either directly or indirectly through the Notch signaling pathway.
Keywords: Embryo implantation; Endometrial adhesion; Endometrial epithelial cell; Hippo pathway; MAML1; Notch pathway; Trophoblast cell.
Conflict of interest statement
The authors report no competing interests.
Figures

Similar articles
-
Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity.Reprod Biol Endocrinol. 2020 Jun 29;18(1):66. doi: 10.1186/s12958-020-00624-w. Reprod Biol Endocrinol. 2020. PMID: 32600462 Free PMC article.
-
Jagged1 regulates endometrial receptivity in both humans and mice.FASEB J. 2021 Aug;35(8):e21784. doi: 10.1096/fj.202100590R. FASEB J. 2021. PMID: 34252231
-
Tripeptidyl peptidase I promotes human endometrial epithelial cell adhesive capacity implying a role in receptivity.Reprod Biol Endocrinol. 2020 Dec 14;18(1):124. doi: 10.1186/s12958-020-00682-0. Reprod Biol Endocrinol. 2020. PMID: 33317560 Free PMC article.
-
Implantation in the baboon: endometrial responses.Semin Reprod Endocrinol. 1999;17(3):257-65. doi: 10.1055/s-2007-1016233. Semin Reprod Endocrinol. 1999. PMID: 10797944 Review.
-
Using organoids to investigate human endometrial receptivity.Front Endocrinol (Lausanne). 2023 Aug 24;14:1158515. doi: 10.3389/fendo.2023.1158515. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37693361 Free PMC article. Review.
Cited by
-
The effect of obesity on uterine receptivity is mediated by endometrial extracellular vesicles that control human endometrial stromal cell decidualization and trophoblast invasion.J Extracell Biol. 2023 Jul 18;2(7):e103. doi: 10.1002/jex2.103. eCollection 2023 Jul. J Extracell Biol. 2023. PMID: 38939074 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

