Vaccines - safety in pregnancy
- PMID: 33773923
- PMCID: PMC7992376
- DOI: 10.1016/j.bpobgyn.2021.02.002
Vaccines - safety in pregnancy
Abstract
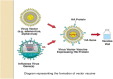
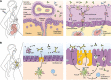
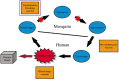
Vaccination during pregnancy is important for active immunity of the mother against serious infectious diseases, and also for passive immunity of the neonate to infectious diseases with high morbidity and mortality. As a rule, live vaccines are contraindicated during pregnancy as they may cause fetal viremia/bacteremia. Inactivated vaccines are generally safe. Vaccines safe to be administered to all pregnant ladies are tetanus toxoid (TT; tetanus, diphtheria, acellular pertussis (Tdap) and Flu vaccines. During pre-pregnancy counselling, vaccination for MMR (measles, mumps, and rubella) should be offered, with an advice to avoid pregnancy for a month. All pregnant mothers should receive TT and Tdap vaccination during the third trimester. Flu vaccine can be given to all mothers at any gestation, and if not offered during pregnancy, it can be given postpartum. Vaccinations that should be offered to women if at high risk of exposure are for hepatitis A and B, pneumococcal, meningococcal, yellow fever, Japanese encephalitis (JE), polio, typhoid, and cholera infections. Vaccines to be given only for post-exposure prophylaxis (PEP) are smallpox, rabies, and anthrax. Postpartum women should be offered human papillomavirus (HPV) vaccination. If not immunized earlier, they should be offered MMR, Tdap, and Flu vaccines. Future vaccines being developed are for malaria, Zika virus, respiratory syncytial virus (RSV), group B streptococcus, CMV, and COVID-19 (SARS-Cov-2).
Keywords: Immunization; Pregnancy; Safety in pregnancy; Vaccination.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest Authors Dr mala arora has no conflict of interest to declare Dr rama lakshmi has no conflict of interest to declare
Figures
References
-
- Bonhoeffe J., Kochhar S., Kirschfeld S., Heath P.T., Jones J.B., Bauwens J.,., et al. Global alignment of Immunization safety assessment in Pregnancy -The GAIA project. Vaccine. 2016;34:5993–5997. - PubMed
-
- Jones C.E., Calvert A., Doare K.L. Vaccination in pregnancy-recent developments, ESPID reports and reviews. Pediatr Infect Dis J. 2018;37(Number 2):191–193. - PubMed
-
- WHO Position Paper- Tetanus vaccines 2017. http://www.who.int/immunization/documents/positionpapers available at:
-
- Centres for Disease Control and Prevention Epidemiology and Prevention of vaccine-Preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/flu.html [Chapter 12]: Influenza; available at:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

