Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis
- PMID: 33774059
- PMCID: PMC8310924
- DOI: 10.1016/j.jhep.2021.03.016
Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis
Abstract
Background & aims: Little is known about the composition of intrahepatic immune cells and their contribution to the pathogenesis of primary sclerosing cholangitis (PSC). Herein, we aimed to create an atlas of intrahepatic T cells and thereby perform an in-depth characterization of T cells in inflamed human liver.
Methods: Different single-cell RNA sequencing methods were combined with in silico analyses on intrahepatic and peripheral T cells from patients with PSC (n = 11) and healthy donors (HDs, n = 4). Multi-parameter flow cytometry and functional in vitro experiments were conducted on samples from patients with PSC (n = 24), controls with other liver diseases and HDs.
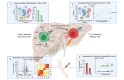
Results: We identified a population of intrahepatic naive-like CD4+ T cells, which was present in all liver diseases tested, but particularly expanded in PSC. This population had a transcriptome and T cell receptor repertoire similar to circulating naive T cells but expressed a set of genes associated with tissue residency. Their periductal location supported the concept of tissue-resident naive-like T cells in livers of patients with PSC. Trajectory inference suggested that these cells had the developmental propensity to acquire a T helper 17 (TH17) polarization state. Functional and chromatin accessibility experiments revealed that circulating naive T cells in patients with PSC were predisposed to polarize towards TH17 cells.
Conclusion: We report the first atlas of intrahepatic T cells in PSC, which led to the identification of a previously unrecognized population of tissue-resident naive-like T cells in the inflamed human liver and to the finding that naive CD4+ T cells in PSC harbour the propensity to develop into TH17 cells.
Lay summary: The composition of intrahepatic immune cells in primary sclerosing cholangitis (PSC) and their contribution to disease pathogenesis is widely unknown. We analysed intrahepatic T cells and identified a previously uncharacterized population of liver-resident CD4+ T cells which are expanded in the livers of patients with PSC compared to healthy liver tissue and other liver diseases. These cells are likely to contribute to the pathogenesis of PSC and could be targeted in novel therapeutic approaches.
Keywords: Atlas; Immune-mediated liver disease; Naive T cells; Primary Sclerosing Cholangitis; Single-cell sequencing; T cells; T(H)17 cells; Tissue residency.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures





References
-
- Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67(6):1298–1323. - PubMed
-
- Boonstra K., Beuers U., Ponsioen C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. - PubMed
-
- Liwinski T., Zenouzi R., John C., Ehlken H., Rühlemann M.C., Bang C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69(4):665–672. - PubMed
-
- Rühlemann M.C., Heinsen F.-A., Zenouzi R., Lieb W., Franke A., Schramm C. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut. 2017;66(4):753–754. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

