Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection
- PMID: 33774082
- PMCID: PMC7992297
- DOI: 10.1016/j.kint.2021.03.009
Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection
Abstract
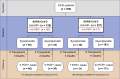
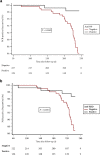
Patients with end stage kidney disease receiving in-center hemodialysis (ICHD) have had high rates of SARS-CoV-2 infection. Following infection, patients receiving ICHD frequently develop circulating antibodies to SARS-CoV-2, even with asymptomatic infection. Here, we investigated the durability and functionality of the immune responses to SARS-CoV-2 infection in patients receiving ICHD. Three hundred and fifty-six such patients were longitudinally screened for SARS-CoV-2 antibodies and underwent routine PCR-testing for symptomatic and asymptomatic infection. Patients were regularly screened for nucleocapsid protein (anti-NP) and receptor binding domain (anti-RBD) antibodies, and those who became seronegative at six months were screened for SARS-CoV-2 specific T-cell responses. One hundred and twenty-nine (36.2%) patients had detectable antibody to anti-NP at time zero, of whom 127 also had detectable anti-RBD. Significantly, at six months, 71/111 (64.0%) and 99/116 (85.3%) remained anti-NP and anti-RBD seropositive, respectively. For patients who retained antibody, both anti-NP and anti-RBD levels were reduced significantly after six months. Eleven patients who were anti-NP seropositive at time zero, had no detectable antibody at six months; of whom eight were found to have SARS-CoV-2 antigen specific T cell responses. Independent of antibody status at six months, patients with baseline positive SARS-CoV-2 serology were significantly less likely to have PCR confirmed infection over the following six months. Thus, patients receiving ICHD mount durable immune responses six months post SARS-CoV-2 infection, with fewer than 3% of patients showing no evidence of humoral or cellular immunity.
Keywords: COVID-19; SARS-CoV-2; hemodialysis; serology.
Copyright © 2021 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barrett J.R., Belij-Rammerstorfer S., Dold C. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

