Maternal preconception PFOS exposure of Drosophila melanogaster alters reproductive capacity, development, morphology and nutrient regulation
- PMID: 33774094
- PMCID: PMC8085153
- DOI: 10.1016/j.fct.2021.112153
Maternal preconception PFOS exposure of Drosophila melanogaster alters reproductive capacity, development, morphology and nutrient regulation
Abstract
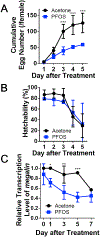
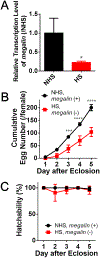
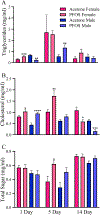
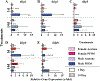
Perfluorooctanesulfonic acid (PFOS) is a persistent synthetic surfactant widely detected in the environment. Developmental PFOS exposures are associated with low birth weight and chronic exposures increase risk for obesity and type 2 diabetes. As an obesogen, PFOS poses a major public health exposure risk and much remains to be understood about the critical windows of exposure and mechanisms impacted, especially during preconception. Here, we leverage evolutionarily conserved pathways and processes in the fruit fly Drosophila melanogaster (wild-type Canton-S and megalin-UAS RNAi transgenic fly lines) to investigate the window of maternal preconception exposure to PFOS on reproductive and developmental toxicity, and examine receptor (megalin)-mediated endocytosis of nutrients and PFOS into the oocyte as a potential mechanism. Preconception exposure to 2 ng PFOS/female resulted in an internal concentration of 0.081 ng/fly over two days post exposure, no mortality and reduced megalin transcription. The number of eggs laid 1-3 days post exposure was reduced and contained 0.018 ng PFOS/egg. Following heat shock, PFOS was significantly reduced in eggs from megalin-knockdown transgenic females. Cholesterol and triglycerides were increased in eggs laid immediately following PFOS exposure by non-heat shocked transgenic females whereas decreased cholesterol and increased protein levels were found in eggs laid by heat shocked transgenic females. Preconception exposure likewise increased cholesterol in early emerging wildtype F1 adults and also resulted in progeny with a substantial developmental delay, a reduction in adult weights, and altered transcription of Drosophila insulin-like peptide genes. These findings support an interaction between PFOS and megalin that interferes with normal nutrient transport during oocyte maturation and embryogenesis, which may be associated with later in life developmental delay and reduced weight.
Keywords: Development; Drosophila melanogaster; Nutrient regulation; Perfluorooctanesulfonic acid (PFOS); Preconception exposure; Reproduction.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S. 2006. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci 119: 2127–2137. - PubMed
-
- Bujo H, Hermann M, Lindstedt KA, Nimpf J, Schneider WJ. 1997. Low density lipoprotein receptor gene family members mediate yolk deposition. The Journal of Nutrition 127: 801S–804S. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

