The role of PPARγ in chemotherapy-evoked pain
- PMID: 33774149
- PMCID: PMC8089062
- DOI: 10.1016/j.neulet.2021.135845
The role of PPARγ in chemotherapy-evoked pain
Abstract
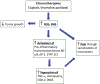
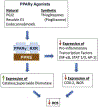
Although millions of people are diagnosed with cancer each year, survival has never been greater thanks to early diagnosis and treatments. Powerful chemotherapeutic agents are highly toxic to cancer cells, but because they typically do not target cancer cells selectively, they are often toxic to other cells and produce a variety of side effects. In particular, many common chemotherapies damage the peripheral nervous system and produce neuropathy that includes a progressive degeneration of peripheral nerve fibers. Chemotherapy-induced peripheral neuropathy (CIPN) can affect all nerve fibers, but sensory neuropathies are the most common, initially affecting the distal extremities. Symptoms include impaired tactile sensitivity, tingling, numbness, paraesthesia, dysesthesia, and pain. Since neuropathic pain is difficult to manage, and because degenerated nerve fibers may not grow back and regain normal function, considerable research has focused on understanding how chemotherapy causes painful CIPN so it can be prevented. Due to the fact that both therapeutic and side effects of chemotherapy are primarily associated with the accumulation of reactive oxygen species (ROS) and oxidative stress, this review focuses on the activation of endogenous antioxidant pathways, especially PPARγ, in order to prevent the development of CIPN and associated pain. The use of synthetic and natural PPARγ agonists to prevent CIPN is discussed.
Keywords: Chemotherapy; Hyperalgesia; Neuropathy; PPARγ; Reactive oxygen species.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of interest
None
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

