NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases
- PMID: 33774476
- PMCID: PMC8027773
- DOI: 10.1016/j.redox.2021.101947
NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases
Abstract
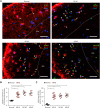
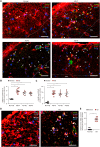
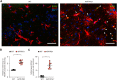
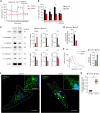
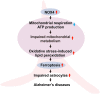
Oxidative stress has been implicated in the pathogenesis of Alzheimer's disease (AD). Mitochondrial dysfunction is linked to oxidative stress and reactive oxygen species (ROS) in neurotoxicity during AD. Impaired mitochondrial metabolism has been associated with mitochondrial dysfunction in brain damage of AD. While the role of NADPH oxidase 4 (NOX4), a major source of ROS, has been identified in brain damage, the mechanism by which NOX4 regulates ferroptosis of astrocytes in AD remains unclear. Here, we show that the protein levels of NOX4 were significantly elevated in impaired astrocytes of cerebral cortex from patients with AD and APP/PS1 double-transgenic mouse model of AD. The levels of 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA), a marker of oxidative stress-induced lipid peroxidation, were significantly also elevated in impaired astrocytes of patients with AD and mouse AD. We demonstrate that the over-expression of NOX4 significantly increases the impairment of mitochondrial metabolism by inhibition of mitochondrial respiration and ATP production via the reduction of five protein complexes in the mitochondrial ETC in human astrocytes. Moreover, the elevation of NOX4 induces oxidative stress by mitochondrial ROS (mtROS) production, mitochondrial fragmentation, and inhibition of cellular antioxidant process in human astrocytes. Furthermore, the elevation of NOX4 increased ferroptosis-dependent cytotoxicity by the activation of oxidative stress-induced lipid peroxidation in human astrocytes. These results suggest that NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in AD.
Keywords: Alzheimer's disease; Ferroptosis; Mitochondrial metabolism; NOX4; Oxidative stress.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

