Developing Brain Glucose Transporters, Serotonin, Serotonin Transporter, and Oxytocin Receptor Expression in Response to Early-Life Hypocaloric and Hypercaloric Dietary, and Air Pollutant Exposures
- PMID: 33774619
- PMCID: PMC8344089
- DOI: 10.1159/000514709
Developing Brain Glucose Transporters, Serotonin, Serotonin Transporter, and Oxytocin Receptor Expression in Response to Early-Life Hypocaloric and Hypercaloric Dietary, and Air Pollutant Exposures
Abstract
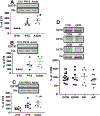
Perturbed maternal diet and prenatal exposure to air pollution (AP) affect the fetal brain, predisposing to postnatal neurobehavioral disorders. Glucose transporters (GLUTs) are key in fueling neurotransmission; deficiency of the neuronal isoform GLUT3 culminates in autism spectrum disorders. Along with the different neurotransmitters, serotonin (5-HT) and oxytocin (OXT) are critical for the development of neural connectivity. Serotonin transporter (SERT) modulates synaptic 5-HT levels, while the OXT receptor (OXTR) mediates OXT action. We hypothesized that perturbed brain GLUT1/GLUT3 regulated 5-HT-SERT imbalance, which serves as a contributing factor to postnatal neuropsychiatric phenotypes, with OXT/OXTR providing a counterbalance. Employing maternal diet restriction (intrauterine growth restriction [IUGR]), high-fat (HF) dietary modifications, and prenatal exposure to simulated AP, fetal (E19) murine brain 5-HT was assessed by ELISA with SERT and OXTR being localized by immunohistochemistry and measured by quantitative Western blot analysis. IUGR with lower head weights led to a 48% reduction in male and female fetal brain GLUT3 with no change in GLUT1, when compared to age- and sex-matched controls, with no significant change in OXTR. In addition, a ∼50% (p = 0.005) decrease in 5-HT and SERT concentrations was displayed in fetal IUGR brains. In contrast, despite emergence of microcephaly, exposure to a maternal HF diet or AP caused no significant changes. We conclude that in the IUGR during fetal brain development, reduced GLUT3 is associated with an imbalanced 5-HT-SERT axis. We speculate that these early changes may set the stage for altering the 5HT-SERT neural axis with postnatal emergence of associated neurodevelopmental disorders.
Keywords: Environmental exposure; Fetal brain; Intrauterine growth restriction; Neurodevelopment; Neurotransmitters.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no competing financial interests.
Figures

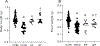



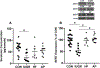

References
-
- Dienel GA. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev. 2019. January 1;99(1):949–1045. - PubMed
-
- Mitanchez D. [Ontogenesis of glucose regulation in neonate and consequences in neonatal management]. Arch Pediatr. 2008. January;15(1):64–74. - PubMed
-
- Takata K, Hirano H, Kasahara M. Transport of glucose across the blood-tissue barriers. Int Rev Cytol. 1997;172:1–53. - PubMed
-
- Khan JY, Rajakumar RA, McKnight RA, Devaskar UP, Devaskar SU. Developmental regulation of genes mediating murine brain glucose uptake. The American journal of physiology. 1999. March;276(3):R892–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

