A first-in-human phase I study of TAS0728, an oral covalent binding inhibitor of HER2, in patients with advanced solid tumors with HER2 or HER3 aberrations
- PMID: 33774767
- PMCID: PMC8426237
- DOI: 10.1007/s10637-021-01104-7
A first-in-human phase I study of TAS0728, an oral covalent binding inhibitor of HER2, in patients with advanced solid tumors with HER2 or HER3 aberrations
Abstract
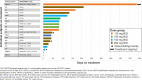
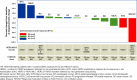
TAS0728 is an oral covalent binding inhibitor of human epidermal growth factor receptor 2 (HER2). A first-in-human open-label, dose-escalation, phase I study (NCT03410927) was initiated to investigate the safety and dose-limiting toxicity (DLT) and to determine the maximum tolerated dose (MTD) and/or recommended phase II dose of TAS0728 in adults with advanced solid tumors with HER2 or HER3 overexpression, amplification or mutation. In total, 19 patients received TAS0728 at escalating doses from 50 to 200 mg BID for 21-day cycles. Following escalation of the dose to 200 mg BID, a total of two DLTs were observed, both cases of Grade 3 diarrhea (lasting >48 h and not responsive to aggressive antidiarrheal treatment). Following de-escalation of the dose to 150 mg BID, another DLT of Grade 3 diarrhea was observed in one patient. Additionally, at 150 mg BID, one patient had a fatal cardiac arrest after receiving 1 cycle (21 days) of TAS0728. The etiology of the cardiac arrest event was not clear, however causal relationship to TAS0728 could not be excluded due to the temporal association observed. Partial responses were observed in 2 of 14 patients evaluable for TAS0728 treatment response. The study was stopped due to unacceptable toxicity during the dose-escalation as the overall risk-benefit ratio no longer favored the dose level being tested, therefore the MTD was not determined. ClinicalTrials.gov registration number: https://clinicaltrials.gov/ct2/show/NCT03410927 ; registered on January 25, 2018.
Keywords: Erb-B2 receptor tyrosine kinase 3; Human epidermal growth factor receptor 2; Neoplasms; Phase I study; TAS0728.
© 2021. The Author(s).
Conflict of interest statement
Figures
References
-
- Irie H, Ito K, Fujioka Y, Oguchi K, Fujioka A, Hashimoto A, Ohsawa H, Tanaka K, Funabashi K, Araki H, Kawai Y, Shimamura T, Wadhwa R, Ohkubo S, Matsuo K. TAS0728, a covalent-binding, HER2-selective kinase inhibitor shows potent antitumor activity in preclinical models. Mol Cancer Ther. 2019;18:733–742. doi: 10.1158/1535-7163.MCT-18-1085. - DOI - PubMed
-
- Collins DM, Conlon NT, Kannan S, Verma CS, Eli LD, Lalani AS, Crown J. Preclinical characteristics of the irreversible pan-HER kinase inhibitor Neratinib compared with Lapatinib: implications for the treatment of HER2-positive and HER2-mutated breast Cancer. Cancers. 2019;11:737. doi: 10.3390/cancers11060737. - DOI - PMC - PubMed
-
- Cortés J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, le Rhun E, Espié M, Kim SB, Schneeweiss A, Sohn JH, Nabholtz JM, Kellokumpu-Lehtinen PL, Taguchi J, Piacentini F, Ciruelos E, Bono P, Ould-Kaci M, Roux F, Joensuu H. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015;16:1700–1710. doi: 10.1016/S1470-2045(15)00373-3. - DOI - PubMed
-
- Harbeck N, Huang CS, Hurvitz S, Yeh DC, Shao Z, Im SA, Jung KH, Shen K, Ro J, Jassem J, Zhang Q, Im YH, Wojtukiewicz M, Sun Q, Chen SC, Goeldner RG, Uttenreuther-Fischer M, Xu B, Piccart-Gebhart M, LUX-Breast 1 study group Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 2016;17:357–366. doi: 10.1016/S1470-2045(15)00540-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous