Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake
- PMID: 33774895
- PMCID: PMC8428832
- DOI: 10.1111/pbi.13589
Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake
Abstract
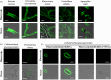
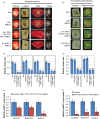
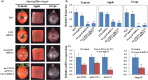
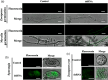
Recent discoveries show that fungi can take up environmental RNA, which can then silence fungal genes through environmental RNA interference. This discovery prompted the development of Spray-Induced Gene Silencing (SIGS) for plant disease management. In this study, we aimed to determine the efficacy of SIGS across a variety of eukaryotic microbes. We first examined the efficiency of RNA uptake in multiple pathogenic and non-pathogenic fungi, and an oomycete pathogen. We observed efficient double-stranded RNA (dsRNA) uptake in the fungal plant pathogens Botrytis cinerea, Sclerotinia sclerotiorum, Rhizoctonia solani, Aspergillus niger and Verticillium dahliae, but no uptake in Colletotrichum gloeosporioides, and weak uptake in a beneficial fungus, Trichoderma virens. For the oomycete plant pathogen, Phytophthora infestans, RNA uptake was limited and varied across different cell types and developmental stages. Topical application of dsRNA targeting virulence-related genes in pathogens with high RNA uptake efficiency significantly inhibited plant disease symptoms, whereas the application of dsRNA in pathogens with low RNA uptake efficiency did not suppress infection. Our results have revealed that dsRNA uptake efficiencies vary across eukaryotic microbe species and cell types. The success of SIGS for plant disease management can largely be determined by the pathogen's RNA uptake efficiency.
Keywords: RNA interference; double-stranded RNA; small RNA; spray-induced gene silencing; uptake efficiency.
© 2021 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Abdellatef, E. , Will, T. , Koch, A. , Imani, J. , Vilcinskas, A. and Kogel, K.H. (2015) Silencing the expression of the salivary sheath protein causes transgenerational feeding suppression in the aphid Sitobion avenae . Plant Biotechnol. J. 13, 849–857. - PubMed
-
- Avrova, A.O. , Boevink, P.C. , Young, V. , Grenville‐Briggs, L.J. , van West, P. , Birch, P.R. and Whisson, S.C. (2008) A novel Phytophthora infestans haustorium‐specific membrane protein is required for infection of potato. Cell. Microbiol. 10, 2271–2284. - PubMed
-
- Baum, J.A. , Bogaert, T. , Clinton, W. , Heck, G.R. , Feldmann, P. , Ilagan, O. , Johnson, S. et al. (2007) Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 25, 1322–1326. - PubMed
-
- Bebber, D.P. and Gurr, S.J. (2015) Crop‐destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 74, 62–64. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

