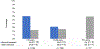Use of Backup Stem Cells for Stem Cell Boost and Second Transplant in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Transplantation
- PMID: 33775587
- PMCID: PMC8113075
- DOI: 10.1016/j.jtct.2021.02.026
Use of Backup Stem Cells for Stem Cell Boost and Second Transplant in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Transplantation
Abstract
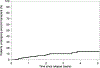
Autologous hematopoietic stem cell transplantation (ASCT) is a standard treatment for multiple myeloma (MM). Consensus guidelines recommend collecting sufficient stem cells in case there is a need for stem cell boost for delayed/poor engraftment or for future second ASCT. However, collecting and storing backup stem cells in all patients requires significant resources and cost, and the rates of backup stem cell utilization are not well studied. We sought to examine the utilization of backup stem cells (BSCs) in patients with MM undergoing ASCT. Patients with MM aged ≥18 years old who underwent first ASCT at our institution from January 2010 through December 2015 and collected sufficient stem cells for at least 2 transplants were included in this single-center retrospective study. This timeframe was selected to allow for adequate follow-up. A total of 393 patients were included. The median age was 58 years (range, 25-73). After a median follow-up of 6 years, the median progression-free survival (PFS) of the cohort was 3 years. Sixty-one percent (n = 240) of patients progressed or relapsed. Chemotherapy-based mobilization was used in almost all patients (98%). The median total CD34+ cells collected was 18.2 × 106/kg (range, 3.4-112.4). A median of 5.7 × 106 CD34+ cells/kg (range, 1.8-41.9) was infused during the first ASCT, and a median of 10.1 × 106 CD34+ cells/kg (range, 1.5-104.5) was cryopreserved for future use. Of the patients, 6.9% (n = 27) used backup stem cells, with 2.3% (n = 10) using them for stem cell boost, 4.6% (n = 18) for a second salvage ASCT, including 1 patient for both stem cell boost and second ASCT. Rates of backup stem cell use among patients aged <60, 60-69, and ≥70 years were 7.8%, 5.7%, and 5.9%, respectively. There was a trend toward higher rates of backup stem cell use for second ASCT in patients who were younger, had suboptimal disease control at time of first ASCT, and longer PFS. The median dose of stem cell boost given was 5.6 × 106 CD34+ cells/kg (range, 1.9-20). The median time from stem cell boost to neutrophil, hemoglobin, and platelet engraftment was 4 (range, 2-11), 15 (range, 4-34), and 12 (range, 0-34) days, respectively. Lower CD34+ dose and older age at time of ASCT predicted need for stem cell boost. With new salvage therapies for relapsed MM, the rates of second ASCT are very low. The low rates of use suggest that institutional policies regarding universal BSC collection and long-term storage should be reassessed and individualized. However, need for stem cell boost in 2.3% of patients may present a challenge to that.
Keywords: Autologous stem cell transplantation; Cryopreservation; Delayed engraftment; Mobilization; Multiple myeloma; Nonengraftment; Salvage transplantation.
Copyright © 2021 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Benboubker L et al. Lenalidomide and Dexamethasone in Transplant-Ineligible Patients with Myeloma. N. Engl. J. Med 371, 906–917 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical