The role of RNA processing and regulation in metastatic dormancy
- PMID: 33775829
- PMCID: PMC8464634
- DOI: 10.1016/j.semcancer.2021.03.020
The role of RNA processing and regulation in metastatic dormancy
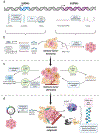
Abstract
Tumor dormancy is a major contributor to the lethality of metastatic disease, especially for cancer patients who develop metastases years-to-decades after initial diagnosis. Indeed, tumor cells can disseminate during early disease stages and persist in new microenvironments at distal sites for months, years, or even decades before initiating metastatic outgrowth. This delay between primary tumor remission and metastatic relapse is known as "dormancy," during which disseminated tumor cells (DTCs) acquire quiescent states in response to intrinsic (i.e., cellular) and extrinsic (i.e., microenvironmental) signals. Maintaining dormancy-associated phenotypes requires DTCs to activate transcriptional, translational, and post-translational mechanisms that engender cellular plasticity. RNA processing is emerging as an essential facet of cellular plasticity, particularly with respect to the initiation, maintenance, and reversal of dormancy-associated phenotypes. Moreover, dysregulated RNA processing, particularly that associated with alternative RNA splicing and expression of noncoding RNAs (ncRNAs), can occur in DTCs to mediate intrinsic and extrinsic metastatic dormancy. Here we review the pathophysiological impact of alternative RNA splicing and ncRNAs in promoting metastatic dormancy and disease recurrence in human cancers.
Keywords: Alternative splicing; Cancer stem cells; Epithelial-mesenchymal transition; Metastatic dormancy; Noncoding RNA; RNA processing; lncRNA; microRNA.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests
The authors declare that no conflict of interest exists.
My colleagues and I declare that we have no competing financial interests
Figures

Similar articles
-
Unveiling cancer dormancy: Intrinsic mechanisms and extrinsic forces.Cancer Lett. 2024 Jun 1;591:216899. doi: 10.1016/j.canlet.2024.216899. Epub 2024 Apr 21. Cancer Lett. 2024. PMID: 38649107 Review.
-
The Relationship Between Dormant Cancer Cells and Their Microenvironment.Adv Cancer Res. 2016;132:45-71. doi: 10.1016/bs.acr.2016.07.002. Epub 2016 Aug 25. Adv Cancer Res. 2016. PMID: 27613129 Free PMC article. Review.
-
The pan-therapeutic resistance of disseminated tumor cells: Role of phenotypic plasticity and the metastatic microenvironment.Semin Cancer Biol. 2020 Feb;60:138-147. doi: 10.1016/j.semcancer.2019.07.021. Epub 2019 Jul 31. Semin Cancer Biol. 2020. PMID: 31376430 Free PMC article. Review.
-
PRRX1 Regulates Cellular Phenotype Plasticity and Dormancy of Head and Neck Squamous Cell Carcinoma Through miR-642b-3p.Neoplasia. 2019 Feb;21(2):216-229. doi: 10.1016/j.neo.2018.12.001. Epub 2019 Jan 7. Neoplasia. 2019. PMID: 30622052 Free PMC article.
-
Microenvironmental Regulation of Dormancy in Breast Cancer Metastasis: "An Ally that Changes Allegiances".Adv Exp Med Biol. 2025;1464:373-395. doi: 10.1007/978-3-031-70875-6_18. Adv Exp Med Biol. 2025. PMID: 39821034 Review.
Cited by
-
An Immune-Related lncRNA Signature to Predict the Biochemical Recurrence and Immune Landscape in Prostate Cancer.Int J Gen Med. 2021 Nov 30;14:9031-9049. doi: 10.2147/IJGM.S336757. eCollection 2021. Int J Gen Med. 2021. PMID: 34876840 Free PMC article.
-
Awakening of Dormant Breast Cancer Cells in the Bone Marrow.Cancers (Basel). 2023 Jun 1;15(11):3021. doi: 10.3390/cancers15113021. Cancers (Basel). 2023. PMID: 37296983 Free PMC article. Review.
-
How circulating tumor cluster biology contributes to the metastatic cascade: from invasion to dissemination and dormancy.Cancer Metastasis Rev. 2023 Dec;42(4):1133-1146. doi: 10.1007/s10555-023-10124-z. Epub 2023 Jul 13. Cancer Metastasis Rev. 2023. PMID: 37442876 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J Clin 70(1) (2020) 7–30. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin 68(6) (2018) 394–424. - PubMed
-
- Hanahan D, Weinberg RA, The hallmarks of cancer, Cell 100(1) (2000) 57–70. - PubMed
-
- Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

