Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship
- PMID: 33775895
- PMCID: PMC8464630
- DOI: 10.1016/j.cgh.2021.03.026
Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship
Abstract
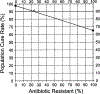
We provide a primer to assist in the difficult transition of Helicobacter pylori therapy guidelines to those that adhere to the principles of antimicrobial stewardship. This transition will entail abandonment of many of the principles that heretofore formed the basis of treatment guidelines and recommendations. The goals of antimicrobial stewardship include optimization of the use of antibiotics while reducing antimicrobial resistance. The critical outcome measure is absolute cure rate which largely restricts comparative trials to those which reliably produce high cure rates (eg, ∼95%). Therapies that fail to achieve at least a 90% cure rate should be abandoned as unacceptable. Because only optimized therapies should be prescribed, guidance on the principles and practices of optimization will we required. Therapies that contain antibiotics which do not contribute to outcome should be eliminated. Surveillance, one of the fundamental elements of antimicrobial stewardship, must be done to provide ongoing assurance that the recommended therapies remain effective. It is yet not widely recognized when utilizing otherwise highly successful therapies, the routine test of cure data is an indirect, surrogate method for susceptibility testing. To systematically guide therapy, test of cure data should be collected, shared and integrated into local antimicrobial stewardship programs to provide guidance regarding best practices to both prescribers and public health individuals. Treatment recommendations should be compatible with those of the American Society of Infectious Disease white paper on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens which include criteria for ethical active-controlled superiority studies of antibacterial agents.
Keywords: Antimicrobial Stewardship; Guidance; Helicobacter pylori; Susceptibility Testing; Test of Cure; Therapy.
Published by Elsevier Inc.
Conflict of interest statement
Figures
References
-
- Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol. Infect 2017;23:793–798. - PubMed
-
- World Health O. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. Geneva: World Health Organization, 2019. https://creativecommons.org/licenses/by-nc-sa/3.0/igo. Assessed 7/15/2020. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

