Membrane-associated mucins of the human ocular surface in health and disease
- PMID: 33775913
- PMCID: PMC8328898
- DOI: 10.1016/j.jtos.2021.03.003
Membrane-associated mucins of the human ocular surface in health and disease
Abstract
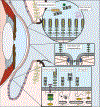
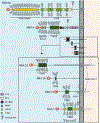
Mucins are a family of high molecular weight, heavily-glycosylated proteins produced by wet epithelial tissues, including the ocular surface epithelia. Densely-packed O-linked glycan chains added post-translationally confer the biophysical properties of hydration, lubrication, anti-adhesion and repulsion. Membrane-associated mucins (MAMs) are the distinguishing components of the mucosal glycocalyx. At the ocular surface, MAMs maintain wetness, lubricate the blink, stabilize the tear film, and create a physical barrier to the outside world. In addition, it is increasingly appreciated that MAMs function as cell surface receptors that transduce information from the outside to the inside of the cell. Recently, our team published a comprehensive review/perspectives article for molecular scientists on ocular surface MAMs, including previously unpublished data and analyses on two new genes MUC21 and MUC22, as well as new MAM functions and biological roles, comparing human and mouse (PMID: 31493487). The current article is a refocus for the audience of The Ocular Surface. First, we update the gene and protein information in a more concise form, and include a new section on glycosylation. Next, we discuss biological roles, with some new sections and further updating from our previous review. Finally, we provide a new chapter on MAM involvement in ocular surface disease. We end this with discussion of an emerging mechanism responsible for damage to the epithelia and their mucosal glycocalyces: the unfolded protein response (UPR). The UPR offers a novel target for therapeutic intervention.
Keywords: Barrier function; Glycocalyx; Membrane-associated mucin; Mucosal epithelia; O-linked glycosylation; Ocular surface; Oxidative stress; Signal transduction; Unfolded protein response.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
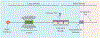
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

