Multiple Ways for Stress Sensing and Regulation of the Endoplasmic Reticulum-stress Sensors
- PMID: 33775971
- PMCID: PMC10511038
- DOI: 10.1247/csf.21015
Multiple Ways for Stress Sensing and Regulation of the Endoplasmic Reticulum-stress Sensors
Abstract
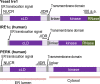
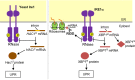
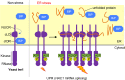
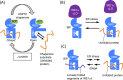
Dysfunction of the endoplasmic reticulum (ER), so-called ER stress, is accompanied with accumulation of unfolded proteins in the ER. Eukaryotic cells commonly have an ER-located transmembrane protein, Ire1, which triggers cellular protective events against ER stress. In animal cells, PERK and ATF6 also initiate the ER-stress response. As a common strategy to control the activity of these ER-stress sensors, an ER-resident molecular chaperone, BiP, serves as their negative regulator, and dissociates from them in response to ER stress. Although it sounds reasonable that unfolded proteins and Ire1 compete for BiP association, some publications argue against this competition model. Moreover, yeast Ire1 (and possibly also the mammalian major Ire1 paralogue IRE1α) directly detects ER-accumulated unfolded proteins, and subsequently oligomerizes for its further activation. Apart from protein misfolding, the saturation of membrane phospholipids is another outcome of ER-stressing stimuli, which is sensed by the transmembrane domain of Ire1. This review describes the canonical and up-to-date insights concerning stress-sensing and regulatory mechanisms of yeast Ire1 and metazoan ER-stress sensors.Key words: endoplasmic reticulum, stress, unfolded protein response, molecular chaperone.
Keywords: endoplasmic reticulum; molecular chaperone; stress; unfolded protein response.
Figures



References
-
- Ali, M.M.U., Bagratuni, T., Davenport, E.L., Nowak, P.R., Silva-Santisteban, M.C., Hardcastle, A., McAndrews, C., Rowlands, M.G., Morgan, G.J., Aherne, W., Collins, I., Davies, F.E., and Pearl, L.H.. 2011. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J., 30: 894–905. - PMC - PubMed
-
- Bashir, S., Banday, M., Qadri, O., Bashir, A., Hilal, N., Nida-I-Fatima, Rader, S., and Fazili, K.M.. 2020. The molecular mechanism and functional diversity of UPR signaling sensor IRE1. Life Sci., 265: 118740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

