Pyroptosis: mechanisms and diseases
- PMID: 33776057
- PMCID: PMC8005494
- DOI: 10.1038/s41392-021-00507-5
Pyroptosis: mechanisms and diseases
Abstract
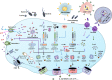
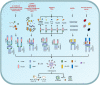
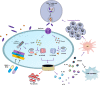
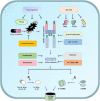
Currently, pyroptosis has received more and more attention because of its association with innate immunity and disease. The research scope of pyroptosis has expanded with the discovery of the gasdermin family. A great deal of evidence shows that pyroptosis can affect the development of tumors. The relationship between pyroptosis and tumors is diverse in different tissues and genetic backgrounds. In this review, we provide basic knowledge of pyroptosis, explain the relationship between pyroptosis and tumors, and focus on the significance of pyroptosis in tumor treatment. In addition, we further summarize the possibility of pyroptosis as a potential tumor treatment strategy and describe the side effects of radiotherapy and chemotherapy caused by pyroptosis. In brief, pyroptosis is a double-edged sword for tumors. The rational use of this dual effect will help us further explore the formation and development of tumors, and provide ideas for patients to develop new drugs based on pyroptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

