Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy
- PMID: 33776489
- PMCID: PMC7987268
- DOI: 10.2147/JEP.S267383
Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy
Abstract
Cisplatin and other platinum-based chemotherapeutic drugs have been used extensively for the treatment of human cancers such as bladder, blood, breast, cervical, esophageal, head and neck, lung, ovarian, testicular cancers, and sarcoma. Cisplatin is commonly administered intravenously as a first-line chemotherapy for patients suffering from various malignancies. Upon absorption into the cancer cell, cisplatin interacts with cellular macromolecules and exerts its cytotoxic effects through a series of biochemical mechanisms by binding to Deoxyribonucleic acid (DNA) and forming intra-strand DNA adducts leading to the inhibition of DNA synthesis and cell growth. Its primary molecular mechanism of action has been associated with the induction of both intrinsic and extrinsic pathways of apoptosis resulting from the production of reactive oxygen species through lipid peroxidation, activation of various signal transduction pathways, induction of p53 signaling and cell cycle arrest, upregulation of pro-apoptotic genes/proteins, and down-regulation of proto-oncogenes and anti-apoptotic genes/proteins. Despite great clinical outcomes, many studies have reported substantial side effects associated with cisplatin monotherapy, while others have shown substantial drug resistance in some cancer patients. Hence, new formulations and several combinational therapies with other drugs have been tested for the purpose of improving the clinical utility of cisplatin. Therefore, this review provides a comprehensive understanding of its molecular mechanisms of action in cancer therapy and discusses the therapeutic approaches to overcome cisplatin resistance and side effects.
Keywords: cancer treatment; cisplatin; combination therapy; molecular mechanisms of action.
© 2021 Tchounwou et al.
Conflict of interest statement
All authors have declared that they do not have any competing and/or financial interests for this work.
Figures
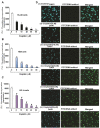
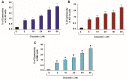
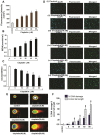

References
-
- Alderden RA, Hall MD, Hambley TW. The discovery and development of cisplatin. J Chem Educ. 2006;83(5):728. doi: 10.1021/ed083p728 - DOI
-
- IARC, International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42; SUPPLEMENT 7. IARC Monogr Eval Carcinog RISKS TO HUMANS - Overall Eval Carcinog An Update IARC Monographs; 1987. - PubMed
-
- The Department of Chemistry at the University of Akron. Akron the chemical database; 2009. Available from: https://pubchem.ncbi.nlm.gov/compound/cis-Platin . Accessed March 04, 2021.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

