Circuit Mechanisms of L-DOPA-Induced Dyskinesia (LID)
- PMID: 33776634
- PMCID: PMC7988225
- DOI: 10.3389/fnins.2021.614412
Circuit Mechanisms of L-DOPA-Induced Dyskinesia (LID)
Abstract
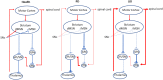
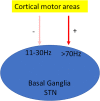
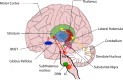
L-DOPA is the criterion standard of treatment for Parkinson disease. Although it alleviates some of the Parkinsonian symptoms, long-term treatment induces L-DOPA-induced dyskinesia (LID). Several theoretical models including the firing rate model, the firing pattern model, and the ensemble model are proposed to explain the mechanisms of LID. The "firing rate model" proposes that decreasing the mean firing rates of the output nuclei of basal ganglia (BG) including the globus pallidus internal segment and substantia nigra reticulata, along the BG pathways, induces dyskinesia. The "firing pattern model" claimed that abnormal firing pattern of a single unit activity and local field potentials may disturb the information processing in the BG, resulting in dyskinesia. The "ensemble model" described that dyskinesia symptoms might represent a distributed impairment involving many brain regions, but the number of activated neurons in the striatum correlated most strongly with dyskinesia severity. Extensive evidence for circuit mechanisms in driving LID symptoms has also been presented. LID is a multisystem disease that affects wide areas of the brain. Brain regions including the striatum, the pallidal-subthalamic network, the motor cortex, the thalamus, and the cerebellum are all involved in the pathophysiology of LID. In addition, although both amantadine and deep brain stimulation help reduce LID, these approaches have complications that limit their wide use, and a novel antidyskinetic drug is strongly needed; these require us to understand the circuit mechanism of LID more deeply.
Keywords: L-DOPA induced dyskinesia; Parkinson’s disease; firing pattern; firing rate; neuronal oscillation.
Copyright © 2021 Yang, Zhao, Wang, Zeng, Luo and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alegre M., Alonso-Frech F., Rodriguez-Oroz M. C., Guridi J., Zamarbide I., Valencia M., et al. (2005). Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur. J. Neurosci. 22 2315–2324. 10.1111/j.1460-9568.2005.04409.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

