Intraoperative Characterization of Subthalamic Nucleus-to-Cortex Evoked Potentials in Parkinson's Disease Deep Brain Stimulation
- PMID: 33776665
- PMCID: PMC7990794
- DOI: 10.3389/fnhum.2021.590251
Intraoperative Characterization of Subthalamic Nucleus-to-Cortex Evoked Potentials in Parkinson's Disease Deep Brain Stimulation
Abstract
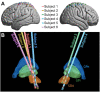
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is a clinically effective tool for treating medically refractory Parkinson's disease (PD), but its neural mechanisms remain debated. Previous work has demonstrated that STN DBS results in evoked potentials (EPs) in the primary motor cortex (M1), suggesting that modulation of cortical physiology may be involved in its therapeutic effects. Due to technical challenges presented by high-amplitude DBS artifacts, these EPs are often measured in response to low-frequency stimulation, which is generally ineffective at PD symptom management. This study aims to characterize STN-to-cortex EPs seen during clinically relevant high-frequency STN DBS for PD. Intraoperatively, we applied STN DBS to 6 PD patients while recording electrocorticography (ECoG) from an electrode strip over the ipsilateral central sulcus. Using recently published techniques, we removed large stimulation artifacts to enable quantification of STN-to-cortex EPs. Two cortical EPs were observed - one synchronized with DBS onset and persisting during ongoing stimulation, and one immediately following DBS offset, here termed the "start" and the "end" EPs respectively. The start EP is, to our knowledge, the first long-latency cortical EP reported during ongoing high-frequency DBS. The start and end EPs differ in magnitude (p < 0.05) and latency (p < 0.001), and the end, but not the start, EP magnitude has a significant relationship (p < 0.001, adjusted for random effects of subject) to ongoing high gamma (80-150 Hz) power during the EP. These contrasts may suggest mechanistic or circuit differences in EP production during the two time periods. This represents a potential framework for relating DBS clinical efficacy to the effects of a variety of stimulation parameters on EPs.
Keywords: Parkinson’s disease; deep brain stimulation; electrocorticography; evoked potential; high-frequency stimulation; subthalamic nucleus.
Copyright © 2021 Levinson, Caldwell, Cronin, Houston, Perlmutter, Weaver, Herron, Ojemann and Ko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

