Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing
- PMID: 33776767
- PMCID: PMC7988345
- DOI: 10.3389/fphar.2021.625678
Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing
Abstract
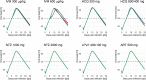
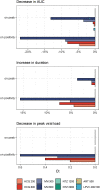
Several repurposed drugs are currently under investigation in the fight against coronavirus disease 2019 (COVID-19). Candidates are often selected solely by their effective concentrations in vitro, an approach that has largely not lived up to expectations in COVID-19. Cell lines used in in vitro experiments are not necessarily representative of lung tissue. Yet, even if the proposed mode of action is indeed true, viral dynamics in vivo, host response, and concentration-time profiles must also be considered. Here we address the latter issue and describe a model of human SARS-CoV-2 viral kinetics with acquired immune response to investigate the dynamic impact of timing and dosing regimens of hydroxychloroquine, lopinavir/ritonavir, ivermectin, artemisinin, and nitazoxanide. We observed greatest benefits when treatments were given immediately at the time of diagnosis. Even interventions with minor antiviral effect may reduce host exposure if timed correctly. Ivermectin seems to be at least partially effective: given on positivity, peak viral load dropped by 0.3-0.6 log units and exposure by 8.8-22.3%. The other drugs had little to no appreciable effect. Given how well previous clinical trial results for hydroxychloroquine and lopinavir/ritonavir are explained by the models presented here, similar strategies should be considered in future drug candidate prioritization efforts.
Keywords: COVID-19; disease modeling; drug repurposing; pharmacometrics; viral kinetics.
Copyright © 2021 Kern, Schöning, Chaccour and Hammann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arshad U., Pertinez H., Box H., Tatham L., Rajoli R. K. R., Curley P., et al. (2020). Prioritization of anti-SARS-cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin. Pharmacol. Ther. 108, 775–790. 10.1002/cpt.1909 - DOI - PMC - PubMed
-
- Balderas-Acata J. I., Ríos-Rogríguezbueno E. P., Pérez-Becerril F., Espinosa-Martínez C., Burke-Fraga V., Parra M. G.-D. L. (2011). Bioavailability of two oral-suspension formulations of a single dose of nitazoxanide 500 mg: an open-label, randomized-sequence, two-period crossover, comparison in healthy fasted Mexican adult volunteers. J Bioequiv Availab 3, 43–47. 10.1016/j.clinthera.2009.08.004 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

