Reduction of Allergic Lung Disease by Mucosal Application of Toxoplasma gondii-Derived Molecules: Possible Role of Carbohydrates
- PMID: 33776987
- PMCID: PMC7988086
- DOI: 10.3389/fimmu.2020.612766
Reduction of Allergic Lung Disease by Mucosal Application of Toxoplasma gondii-Derived Molecules: Possible Role of Carbohydrates
Abstract
Background: The hygiene hypothesis suggests a link between parasitic infections and immune disorders, such as allergic diseases. We previously showed that infection with Toxoplasma gondii or systemic application of T. gondii tachyzoites lysate antigen (TLA) in a prophylactic, but not therapeutic protocol, prevented allergic airway inflammation in mice. Here we tested the effect of prophylactic and therapeutic application of TLA via the mucosal route.
Methods: Mice were intranasally treated with TLA either i) prior to sensitization, ii) during sensitization and challenge, or iii) after sensitization with ovalbumin (OVA). Recruitment of inflammatory cells to the lung, cytokine levels in restimulated lung and spleen cell cultures as well as levels of OVA-specific antibodies in serum were measured. In parallel, the effect of native TLA, heat-inactivated (hiTLA) or deglycosylated TLA (dgTLA) on sensitized splenocytes was evaluated ex vivo.
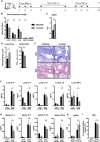
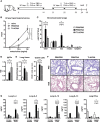
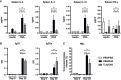
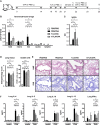
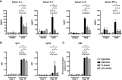
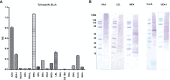
Results: When applied together with OVA i) during systemic sensitization and local challenge or ii) exclusively during local challenge, TLA reduced infiltration of eosinophils into the lung, OVA-specific type 2 cytokines in restimulated lung cell cultures, and partially, type 2 cytokines in restimulated spleen cell cultures in comparison to allergic controls. No beneficial effect was observed when TLA was applied prior to the start of sensitization. Analysis of epitope sugars on TLA indicated a high abundance of mannose, fucose, N-acetylglucosamine, and N-acetylgalactosamine. Deglycosylation of TLA, but not heat-inactivation, abolished the potential of TLA to reduce type 2 responses ex vivo, suggesting a significant role of carbohydrates in immunomodulation.
Conclusion: We showed that mucosal application of TLA reduced the development of experimental allergy in mice. The beneficial effects depended on the timing of the application in relation to the time point of sensitization. Not only co-application, but also therapy in sensitized/allergic animals with native TLA reduced local allergic responses. Furthermore, we show that TLA is highly glycosylated and glycoconjugates seem to play a role in anti-allergic effects. In summary, given the powerful modulatory effect that TLA exhibits, understanding its exact mechanisms of action may lead to the development of novel immunomodulators in clinical application.
Keywords: Toxoplasma gondii; allergic airway inflammation; carbohydrates; deglycosylation; hygiene hypothesis; immunomodulation; parasites; tachyzoites lysate antigen.
Copyright © 2021 Korb, Drinić, Wagner, Geissler, Inic-Kanada, Peschke, Joachim, Wiedermann and Schabussova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

