The Multifaceted Role of Th1, Th9, and Th17 Cells in Immune Checkpoint Inhibition Therapy
- PMID: 33777008
- PMCID: PMC7994325
- DOI: 10.3389/fimmu.2021.625667
The Multifaceted Role of Th1, Th9, and Th17 Cells in Immune Checkpoint Inhibition Therapy
Abstract
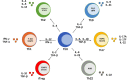
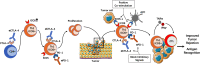
During the last decade, immune checkpoint inhibition (ICI) has become a pillar of cancer therapy. Antibodies targeting CTLA-4 or PD-1/PD-L1 have been approved in several malignancies, with thousands of clinical trials currently underway. While the majority of cancer immunotherapies have traditionally focused on enhancing cytotoxic responses by CD8+ or NK cells, there are clear evidences that CD4+ T cell responses can modulate the immune response against tumors and influence the efficacy of ICI therapy. CD4+ T cells can differentiate into several subsets of helper T cells (Th) or regulatory T cells (Treg), with a wide range of effector and/or regulatory functions. Importantly, different Th subsets may have different and sometimes contrasting roles in the clinical response to ICI therapy, which in addition may vary depending on the organ and tumor niche. In this review, we discuss recent evidence that highlights how ICI therapy impacts Th1, Th9, and Th17 cells and vice versa. These data might be important designing better interventions that unleash the full potential of immune response against cancer.
Keywords: CTLA-4; PD-1; T helper (Th) cell; Th1; Th17; Th9; cancer therapy; immune checkpoint inhibition.
Copyright © 2021 Lee, Lozano-Ruiz, Yang, Fan, Shen and González-Navajas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Busch W. Einfluss von Erysipel. Berliner Klin Wschr (1866) 3:245–6.
-
- Fehleisen F. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch Med Wochenschr (1882) 8:553–4. 10.1055/s-0029-1196806 - DOI
-
- Busch W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berlin Klin Wochenschr (1868) 5:137.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

