Comparison of the Migration Potential through Microperforated Membranes of CD146+ GMSC Population versus Heterogeneous GMSC Population
- PMID: 33777147
- PMCID: PMC7979285
- DOI: 10.1155/2021/5583421
Comparison of the Migration Potential through Microperforated Membranes of CD146+ GMSC Population versus Heterogeneous GMSC Population
Abstract
Background: Guided tissue regeneration (GTR) is a powerful modality for periodontal regeneration, but it blocks the periosteum and gingival stem cells (GMSCs), from supporting periodontal wound by the nutrients, growth factors, and regenerative cells. The microperforated membrane considered a rewarding solution for this major drawback; GMSCs can migrate through a GTR microperforated membrane toward a chemoattractant, with the blocking of other unfavorable epithelial cells and fibroblasts. In the absence of a sole marker for MSC, a homogeneous population of GMSC is difficult to isolate; using CD146 as confirmatory markers for MSC identification, testing the behaviour of such homogeneous population in migration dynamics was the question to answer in this study.
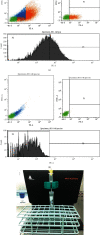
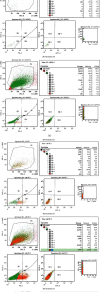
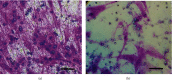
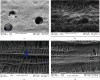
Materials and methods: GMSCs from healthy crown lengthening tissue was isolated (n = 3), its stem cell nature was confirmed, CD146 and CD271 markers were confirmatory markers to confirm homogenous stem cell population, and magnetic sorting was used to isolate GMSC with CD146 markers. A homogenous CD146 population was compared to heterogeneous GMSCs of origin; the population doubling time and MTT test of the two populations were compared. Migration dynamics were examined in a transwell migration chamber through 8 μm perforated polycarbonic acid membrane, and 0.4 μm and 3 μm perforated collagen-coated polytetrafluoroethylene membrane (PTFE) and 10% fetal bovine serum (FBS) were the chemoattractants used in the lower compartment to induce cell migration, were incubated in a humidified environment for 24 hours, then migrated the cell in the lower compartment examined by a light and electron microscope.
Results: GMSCs fulfilled all the minimal criteria of stem cells and showed low signal 10% for CD146 on average and extremely low signal 2% for CD271 on average. Magnetic sorting optimized the signal of CD146 marker to 55%. GMSC CD146 population showed nonstatistically significant shorter population doubling time. CD146 homogeneous population migrated cell numbers were statistically significant compared to the heterogeneous population, through 0.4 μm and 3 μm perforated collagen membrane and 8 μm perforated polycarbonate membrane. Scanning electron microscopy proved the migration of the cells.
Conclusions: A subset of the isolated GMSC showed a CD146 marker, which is considered a dependable confirmatory marker for the stem cells. In terms of GMSC migration through the microperforated membrane, a homogeneous CD146 population migrates more statistically significant than a heterogeneous GMSC population.
Copyright © 2021 Mohamed Al Bahrawy.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures



References
-
- Al Bahrawy M. M., Ghaffar K. K. A., El-Mofty M. S., Rahman A. A. The mutual effect of hyperlipidemia and proinflammatory cytokine related to periodontal infection. Egyptian Journal of Oral and Maxillofacial Surgery. 2011;2(2):87–95. doi: 10.1097/01.OMX.0000405286.13210.e3. - DOI
-
- Haas S. L. The importance of the periosteum and the endosteum in the repair of transplanted bone. Archives of Surgery. 1924;8(2):535–556. doi: 10.1001/archsurg.1924.01120050076004. - DOI
-
- Al Bahrawy M., Gamal A., Ghaffar K. A., Iacono V. In vitro migration dynamics of gingival mesenchymal stem cells through micro perforated membranes. International Journal of Dentistry and Oral Health. 2018;4(5) doi: 10.16966/2378-7090.272. - DOI
-
- Tomar G. B., Srivastava R. K., Gupta N., et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow- derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochemical and Biophysical Research Communications. 2010;393(3):377–383. doi: 10.1016/j.bbrc.2010.01.126. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

